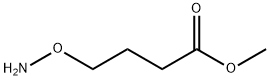
Methyl 4-(aminooxy)butanoate synthesis
- Product Name:Methyl 4-(aminooxy)butanoate
- CAS Number:107903-02-6
- Molecular formula:C5H11NO3
- Molecular Weight:133.15
![Butanoic acid, 4-[(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)oxy]-, methyl ester](/CAS/20210305/GIF/119449-74-0.gif)
119449-74-0
0 suppliers
inquiry

107903-02-6
2 suppliers
inquiry
Yield:107903-02-6 100%
Reaction Conditions:
with methylhydrazine in dichloromethane at -10 - 0; for 1.5 h;
Steps:
3
A solution of methyl γ-phthalimidooxybutyrate (N) (2.75 g, 10.45 mmoles) in dichloromethane (50 mL) was cooled to -10° C. and treated dropwise, with stirring, with methylhydrazine (0.83 mL, 0.72 g, 15.7 mmoles). Stirring was continued for 1.5 h at -10 to 0° C. and the mixture was then filtered. The filtrate was concentrated and the residue, in ethyl acetate (50 mL), was washed with 1:1 saturated sodium chloride:saturated sodium bicarbonate (22 mL). The aqueous layer was washed with ethyl acetate (50 mL) and the combined organic extracts were dried over anhydrous magnesium sulfate and evaporated to give a yellow oil (1.39 g, 100%). 1Hmr (CDCl3, δ): 5.25 (2H, br s, NH2), 3.68 (2H, t, 6.2 Hz, CH2γ), 3.68 (3H, s, OCH3), 2.38 (2H, t, 7.3 Hz, CH2α), 1.92 (2H, quintet, 6.7 Hz, CH2β). 13Cmr (CDCl3, δ): 173.95, 74.78, 51.58, 30.71, 23.65. IR (neat): 3322, 1734 cm-1. Mass spectrum (CI, m/z): 134 (M+1). Calcd. for C5H11NO3: C, 45.09; H, 8.34; N, 10.52. Found: C, 45.02; H, 8.36; N, 10.49.
References:
US2003/232820,2003,A1 Location in patent:Page 14
![Butanoic acid, 4-[(2,5-dioxo-1-pyrrolidinyl)oxy]-, methyl ester](/CAS/20210305/GIF/637008-46-9.gif)
637008-46-9
0 suppliers
inquiry

107903-02-6
2 suppliers
inquiry
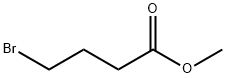
4897-84-1
254 suppliers
$5.00/1g

107903-02-6
2 suppliers
inquiry


![Butanoic acid, 4-[[(diphenylmethylene)amino]oxy]-](/CAS/20210305/GIF/15985-47-4.gif)