
Methyl 4-(benzyloxy)-3-hydroxybenzoate synthesis
- Product Name:Methyl 4-(benzyloxy)-3-hydroxybenzoate
- CAS Number:87687-75-0
- Molecular formula:C15H14O4
- Molecular Weight:258.27
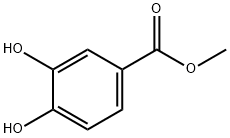
2150-43-8
276 suppliers
$13.43/1gm:

100-39-0
423 suppliers
$10.00/10g

87687-75-0
35 suppliers
inquiry
Yield:87687-75-0 78%
Reaction Conditions:
with hydrogen;potassium carbonate in acetonitrile at 80; for 12 h;
Steps:
23.1 Step 1: Methyl 4-benzyloxy-3-hydroxy-benzoate
To a solution of methyl 3, 4-dihydroxybenzoate (10.00 g, 59.47 mmol, 1.00 eq) and potassium carbonate (8.22 g, 59.47 mmol, 1.00 eq) in acetonitrile (120 niL) was added benzyl bromide (10.17 g, 59.47 mmol, 7.06 niL, 1.00 eq). The mixture was stirred at 80 °C for 12 h under hydrogen atmosphere. LCMS showed that the reaction was completed. The mixture was filtered. The filtrate was concentrated under reduced pressure to give the residue. The residue was diluted with water (100 ml.) and extracted with dichloromethane (200 ml,). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography on silica gel (petroleum ether: ethyl acetate = 20: 1 to 10: 1) to give methyl 4-benzyloxy-3 -hydroxy-benzoate (12.00 g, 46.46 mmol, 78% yield) as a white solid. LCMS: MS (ESI) m/z: 259.1[M+1] +. T-I NMR: (400 MHz, CDCb)5:7.69 - 7.57 (m, 2 H), 7.50 - 7.35 (m, 5 H), 6.97 (d, i 8.4 Hz, 1 H), 5.82 - 5.70 (m, 1 H), 5.19 (s, 2 H), 3.94 - 3.84 (m, 3 H). Chemical Formula: C15H14O4, Molecular Weight: 258.27
References:
WO2021/127443,2021,A1 Location in patent:Paragraph 0781-0787

2150-43-8
276 suppliers
$13.43/1gm:

100-51-6
1385 suppliers
$5.00/100g

87687-75-0
35 suppliers
inquiry

2150-43-8
276 suppliers
$13.43/1gm:

100-44-7
625 suppliers
$13.50/250G

87687-75-0
35 suppliers
inquiry
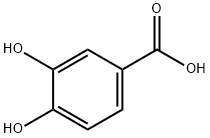
99-50-3
565 suppliers
$10.00/5g

87687-75-0
35 suppliers
inquiry

2150-43-8
276 suppliers
$13.43/1gm:

87687-75-0
35 suppliers
inquiry