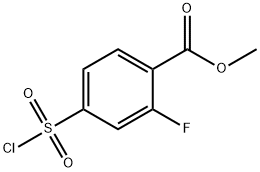
methyl 4-(chlorosulfonyl)-2-fluorobenzoate synthesis
- Product Name:methyl 4-(chlorosulfonyl)-2-fluorobenzoate
- CAS Number:1063733-16-3
- Molecular formula:C8H6ClFO4S
- Molecular Weight:252.65

73792-08-2
120 suppliers
$10.00/1g

1063733-16-3
18 suppliers
inquiry
Yield:1063733-16-3 90%
Reaction Conditions:
Stage #1: methyl 4-amino-2-fluorobenzoatewith hydrogenchloride;sodium nitrite in water at 10; for 0.333333 h;
Stage #2: with sulfur dioxide;acetic acid;copper(l) chloride in water at 0 - 20; for 2 h;
Steps:
1
Step 1: To a solution of methyl 4~amino-2-fluorobenzoate (64.0 g, 378 mmol) in concentrated aq. hydrochloric acid (640 mL) was added aqueous NaN02 solution (28.7 g, 416 mmol, in 50 mL) at 10 °C. After stirring at 10 °C for 20 min, the mixture was added dropwise at 0 °C into a solution of CuCl (375 mg, 3.8 mmol) in HO Ac (500 mL) which was saturated with S02 gas. The resulting mixture was warmed up to room temperature, and stirred for 2 h. The reaction mixture was poured into ice- water and extracted with ethyl acetate. The combined organic phase was washed with saturated aq. NaHC03 solution and then brine, dried over Na2S04, and concentrated under reduced pressure to afford methyl 4-(chlorosulfonyl)-2- fluorobenzoateas a brown oil (86.1g, 90%). 1H NMR (400 MHz, CDC13) d 8.18 (dd, ./= 6.8 Hz, 8.4 Hz, 1H), 7.89 (dd, = 2.0 Hz, 8.4 Hz, 1H), 7.83 (dd, J= 2.0 Hz, 8.8 Hz, 1H), 4.00 (s, 3H).
References:
WO2020/33782,2020,A1 Location in patent:Paragraph 0220

1063733-13-0
3 suppliers
inquiry

1063733-16-3
18 suppliers
inquiry