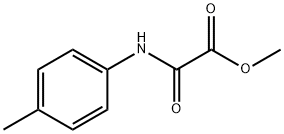
methyl [(4-methylphenyl)carbamoyl]formate synthesis
- Product Name:methyl [(4-methylphenyl)carbamoyl]formate
- CAS Number:80945-63-7
- Molecular formula:C10H11NO3
- Molecular Weight:193.2
![2-Butenedioic acid, 2-[(4-methylphenyl)amino]-, 1,4-dimethyl ester](/CAS/20210305/GIF/87748-33-2.gif)
87748-33-2
0 suppliers
inquiry
![methyl [(4-methylphenyl)carbamoyl]formate](/CAS/20210111/GIF/80945-63-7.gif)
80945-63-7
5 suppliers
inquiry
Yield:80945-63-7 92%
Reaction Conditions:
with tert.-butylhydroperoxide in water;chlorobenzene at 80; for 2 h;
Steps:
Methyl N-phenyloxamate (2a); Typical Procedure
General procedure: In a round-bottomed flask, a mixture of dimethyl 2-(phenylamino)maleate (1a; 0.094 g, 0.4 mmol), 70% aq TBHP (0.131 g, 1mmol), and PhCl (1 mL) was heated in an oil bath at 80 °C for 2h. When the reaction was complete (TLC), the mixture wascooled to r.t. and the reaction was quenched with sat. aq Na2SO3(1 mL). The mixture was extracted with EtOAc, and the organicphase was dried (Na2SO4), filtered, and concentrated underreduced pressure. The residue was purified by column chromatography[silica gel, hexane-EtOAc (8:1)] to give a white solid;yield: 0.063 g (88%);
References:
Adib, Mehdi;Pashazadeh, Rahim;Gohari, Seyed Jamal Adin;Shahsavari, Fatemeh [Synlett,2017,vol. 28,# 12,p. 1481 - 1485] Location in patent:supporting information
99075-46-4
1 suppliers
inquiry
![methyl [(4-methylphenyl)carbamoyl]formate](/CAS/20210111/GIF/80945-63-7.gif)
80945-63-7
5 suppliers
inquiry

67-56-1
739 suppliers
$9.00/25ml

2564-09-2
5 suppliers
inquiry
5602-96-0
25 suppliers
inquiry
![methyl [(4-methylphenyl)carbamoyl]formate](/CAS/20210111/GIF/80945-63-7.gif)
80945-63-7
5 suppliers
inquiry
106-49-0
390 suppliers
$5.00/5G