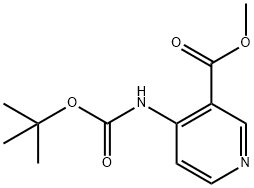
Methyl 4-((tert-butoxycarbonyl)aMino)nicotinate synthesis
- Product Name:Methyl 4-((tert-butoxycarbonyl)aMino)nicotinate
- CAS Number:280115-84-6
- Molecular formula:C12H16N2O4
- Molecular Weight:252.27

67-56-1
741 suppliers
$9.00/25ml
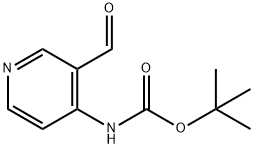
116026-93-8
81 suppliers
$70.00/250mg

280115-84-6
18 suppliers
$518.00/1g
Yield:280115-84-6 96%
Reaction Conditions:
with N-iodo-succinimide;potassium carbonate at 20; for 1 h;
Steps:
8.8B 8B. Formula 504 where R1, R3 and R4 are H; W, Y and Z are -C=: and X is-N=:
To a solution of methanol (100 mL) and N-Boc-4-amino-3-pyridine carboxyaldehyde (8.3 g, 37.3 [MMOL),] were added N-iodosuccinimide (21.0 g, 93.4 [MMOL)] and potassium carbonate (12.9 g, 93.4 [MMOL)] at room temperature. The mixture was stirred for 1 hour and then poured into a saturated Na2S203 solution (100 mL). After further dilution with water (100 mL) the mixture was extracted with dichloromethane (3 X 100 mL). The combined organic layers were dried over sodium sulfate and concentrated under reduced pressure to give the pure desired methyl ester product, 4-tert- butoxycarbonylamino-nicotinic acid methyl ester (9.0 g, 96%). MS (CI) m/e: 253.
References:
WO2003/103575,2003,A2 Location in patent:Page 74
171178-34-0
65 suppliers
$32.00/250mg
18107-18-1
206 suppliers
$33.00/5g

280115-84-6
18 suppliers
$518.00/1g

24424-99-5
823 suppliers
$13.50/25G

16135-36-7
181 suppliers
$9.00/1g

280115-84-6
18 suppliers
$518.00/1g