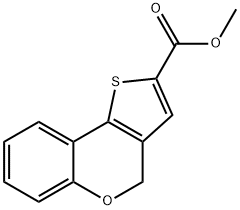
METHYL 4H-(1)-BENZOPYRANO(4 3-B)THIOPHE& synthesis
- Product Name:METHYL 4H-(1)-BENZOPYRANO(4 3-B)THIOPHE&
- CAS Number:126522-01-8
- Molecular formula:C13H10O3S
- Molecular Weight:246.28
Yield:126522-01-8 95%
Reaction Conditions:
with thionyl chloride for 2.5 h;Reflux;
Steps:
Methyl 4H-Thieno[3,2-c]chromene-2-carboxylate (11) [19, 21, 22].
A solution of 4-thieno[3,2-c]-chromene-2-carboxylic acid (10) (0.929 g, 4.0 mmol) in MeOH (6.0 ml) was cooled and treated over 10 min with SOCl2 (0.43 ml, 6.0 mmol). The mixture was refluxed for 2.5 h. The precipitate formed was filtered off and recrystallized from MeOH. Yield 0.936 g (95%). Light-yellow crystals. IR spectrum, ν, cm-1: 1714 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.89 (3H, s, H3); 5.25 (2H, s, CH2); 6.91-7.01 (2H, m, H-6,8); 7.18-7.24 (1H, m, H-7); 7.34 (1H, dd, 3J = 7.7, 4J = 1.5, H-9); 7.51 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 52.2 (CH3); 65.8 (C-4); 117.0 (C-6); 119.5 (C-9b); 122.2 (C-8); 123.6 (C-9); 130.2 (C-7); 130.4 (C-3); 131.6 (C-2); 131.7 (-3a); 139.5 (C-9a); 152.9 (C-5a); 162.6 (C=O).
References:
Bogza, Yu. P.;Katsiel';Sharypova;Tolstikova;Fisyuk [Chemistry of Heterocyclic Compounds,2015,vol. 50,# 12,p. 1712 - 1718][Khim. Geterotsikl. Soedin.,2014,# 12,p. 1862 - 1868,7]

![4H-THIENO[3,2-C]CHROMENE-2-CARBOXYLIC ACID](/CAS/GIF/26268-04-2.gif)