
methyl 5-bromo-2-hydroxy-3-methylbenzoate synthesis
- Product Name:methyl 5-bromo-2-hydroxy-3-methylbenzoate
- CAS Number:40912-71-8
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07
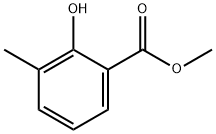
23287-26-5
119 suppliers
$30.00/1g

40912-71-8
18 suppliers
$295.00/1g
Yield:40912-71-8 97%
Reaction Conditions:
with bromine in 1,4-dioxane;dichloromethane at 0 - 20; for 432.5 h;
Steps:
317
Example 317; 317S-Bromo-l-cyclobutoxy-S-methyl-benzoic acid (317)A 25OmL 3 -necked round bottom flask which is fitted with an addition funnel, a N2 inlet and a stopper. The flask is charged with bromine (3.6mL 70.1 lmmol) and DCM (4OmL). A stirring bar is added and stirring is initiated. The reaction flask is immersed in an ice-water bath. After stirring for 15min, the addition funnel is charged with a solution of methyl 2-hydroxy-3- methylbenzoate (1Og, 60.18mmol) in 1,4-dioxane (4OmL). This solution is added to the stirred reaction mixture dropwise over 30min. The addition funnel is then washed with 1,4-dioxane (1OmL). This too is added to the reaction mixture. The reaction mixture is then allowed to slowly warm to ambient temperature. After 18 days, the contents of the reaction flask are transferred to a round bottom flask. The solvent is removed under reduced pressure by pumping to constant weight to give 17.66g of light yellow solid. This solid is triturated with ice-cold MeOH (75mL). The resultant crystals are collected by suction filtration. Air-drying provides white solid (14.26g, 97%).
References:
WO2008/151211,2008,A1 Location in patent:Page/Page column 239
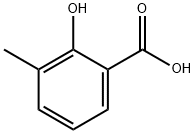
83-40-9
355 suppliers
$5.00/1g

40912-71-8
18 suppliers
$295.00/1g

67-56-1
740 suppliers
$9.00/25ml

36194-82-8
68 suppliers
$18.00/250mg

40912-71-8
18 suppliers
$295.00/1g