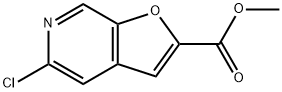
methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate synthesis
- Product Name:methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate
- CAS Number:1315362-16-3
- Molecular formula:C9H6ClNO3
- Molecular Weight:211.6
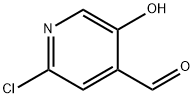
1060804-53-6
55 suppliers
$114.00/100mg
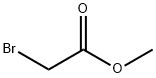
96-32-2
339 suppliers
$15.00/5g
![methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate](/CAS/20180703/GIF/1315362-16-3.gif)
1315362-16-3
11 suppliers
inquiry
Yield:1315362-16-3 79%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 8 h;
Steps:
109B Example 109B: methyl 5 -chlorofuro [2,3 -cjpyridine-2-carboxylate (B- 109)
j00489j To a mixture of 2-chloro-5-hydroxyisonicotinaldehyde (4.0 g, 25 mmol) and methyl 2- bromoacetate (5.8 g, 38 mmol) in N,N-dimethylformamide (40 mL) under nitrogen at room temperature was added potassium carbonate (6.9 g, 50 mmol). The reaction mixture was stirred at 80 °C for 8 hours, then diluted with water (100 mL) and extracted with ethyl acetate (3 x 100 mL). The combined organic layers were washed with brine (2 x 100 mL), dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by silica gel silica gel chromatography [petroleum ether: ethyl acetate = 25:11 to give compound B-109 (4.2 g, 79% yield) as a white solid. LCMS (E): tR=0.68 mm., 212.6 mlz (M+1).
References:
WO2017/69980,2017,A1 Location in patent:Paragraph 00488; 00489
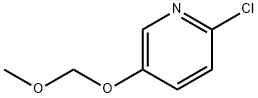
877133-56-7
41 suppliers
$44.00/100mg
![methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate](/CAS/20180703/GIF/1315362-16-3.gif)
1315362-16-3
11 suppliers
inquiry
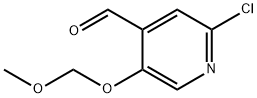
1282516-32-8
9 suppliers
inquiry
![methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate](/CAS/20180703/GIF/1315362-16-3.gif)
1315362-16-3
11 suppliers
inquiry
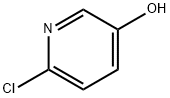
41288-96-4
329 suppliers
$8.00/1g
![methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate](/CAS/20180703/GIF/1315362-16-3.gif)
1315362-16-3
11 suppliers
inquiry

1060804-53-6
55 suppliers
$114.00/100mg
![methyl 5-chlorofuro[2,3-c]pyridine-2-carboxylate](/CAS/20180703/GIF/1315362-16-3.gif)
1315362-16-3
11 suppliers
inquiry