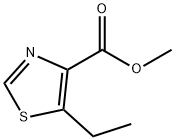
Methyl 5-ethyl-1,3-thiazole-4-carboxylate synthesis
- Product Name:Methyl 5-ethyl-1,3-thiazole-4-carboxylate
- CAS Number:81569-45-1
- Molecular formula:C7H9NO2S
- Molecular Weight:171.22
28942-54-3
37 suppliers
$40.00/250 mg

81569-45-1
9 suppliers
inquiry
Yield: 78%
Reaction Conditions:
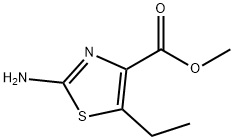
Stage #1:methyl 2-amino-5-ethyl-1,3-thiazole-4-carboxylate with hypophosphorous acid;sodium nitrite in water at 0 - 20; for 3 h;
Stage #2: with sodium hydroxide in water at 0;
Steps:
173.a
The following diazotization step was adapted from Barton, A. et al, J. C.S. Perkin Trans I, 159-164 (1982): A solution of NaN02 (150 mg, 2.17 mmol) in water (1.0 mL) was added dropwise to a stirred, cold (0 °C) solution of methyl 2-amino-5-ethyl-l,3- thiazole-4-carboxylate (186 mg, 1.0 mmol) in 50% H3PO2 (3.2 mL). The mixture was stirred at 0 °C for 1 h and allowed to warm up to room temperature where it stirred further for 2h. After recooling to 0 °C, the mixture was treated slowly with a solution of NaOH (85 mg) in water (10 mL). The mixture was then diluted with saturated NaHCC solution and extracted twice with ether. The organic layers were combined, dried over MgS04 and concentrated to give methyl 5-ethylthiazole-4-carboxylate (i.e., Cap-173, step a) (134 mg, 78%) as an orange oil (85% pure) which was used directly in the next reaction. Rt = 1.58 min (Cond.-MDl); LC/MS: Anal. Calcd. for [M+H]+ C7H10NO2S: 172.05; found: 172.05.
References:
BRISTOL-MYERS SQUIBB COMPANY;PACK, Shawn, K.;TYMONKO, Steven;PATEL, Bharat, P.;NATALIE, JR., Kenneth, J.;BELEMA, Makonen WO2011/59850, 2011, A1 Location in patent:Page/Page column 127-128

1219632-45-7
9 suppliers
inquiry

81569-45-1
9 suppliers
inquiry