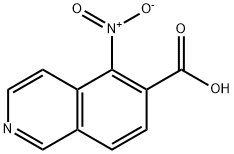
methyl 5-nitroisoquinoline-6-carboxylate synthesis
- Product Name:methyl 5-nitroisoquinoline-6-carboxylate
- CAS Number:1312165-77-7
- Molecular formula:C11H8N2O4
- Molecular Weight:232.19
Yield:-
Reaction Conditions:
Stage #1: 5-nitroisoquinoline-6-carboxylic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0; for 1 h;
Stage #2: methanol for 15 h;
Steps:
1
To a solution of the above compound (1.47 g, 6.75 mmol) in 34 mL of dichloromethane at 0 °C was added N.TV'-dimethylformamide (0.026 mL, 0.34 mmoL) followed by oxalyl chloride (0.65 mL, 7.4 mmol) dropwise. After 1 h, the reaction was concentrated in vacuo and redissolved in methanol. After 15 h, the mixture was concentrated in vacuo, diluted with 10% aqueous sodium bicarbonate, and extracted 3x with dichloromethane. The combined organic fractions were dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified via silica gel chromatography, eluting with 0-25% ethyl acetate in hexanes, to provide methyl 5- nitroisoquinoine-6-carboxylate mat gave proton NMR spectra consistent with theory and a mass ion (ES+) of 233.1 for [M+H]+.
References:
WO2011/75371,2011,A1 Location in patent:Page/Page column 34; 35
607-32-9
234 suppliers
$6.00/5g

1312165-77-7
5 suppliers
inquiry

188121-31-5
55 suppliers
$20.00/250mg

1312165-77-7
5 suppliers
inquiry