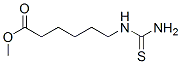
METHYL 6-[(AMINOCARBONOTHIOYL)AMINO]HEXANOATE synthesis
- Product Name:METHYL 6-[(AMINOCARBONOTHIOYL)AMINO]HEXANOATE
- CAS Number:98998-60-8
- Molecular formula:C8H16N2O2S
- Molecular Weight:0
Yield:98998-60-8 78%
Reaction Conditions:
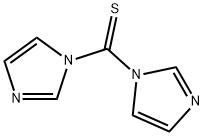
Stage #1: methyl 6-aminohexanoate hydrochloride;1,1'-Thiocarbonyldiimidazolewith triethylamine in tetrahydrofuran at 20; for 12 h;Inert atmosphere;
Stage #2: with ammonium hydroxide in acetonitrile at 60; for 3 h;
Steps:
Methyl 6-thioureidohexanoate (2c).
To a suspension of methyl 6--aminohexanoate (1c, 0.10g, 0.69 mmol) and 1,1'-thiocarbonyldiimidazole (0.23 g, 1.2 mmol, 1.7 eq) in THF (2.75mL)stirring under argon, was added Et3N (0.29 mL,2.1 mmol, 3 eq)and the reaction mixture was left to stir at rt for 12h. The mixture was concentrated before addition of MeCN (1.38 mL, 2.2 mL/mmol)and NH4OH (0.69 mL, 1.1 mL/mmol). The reaction mixture was stirred at 60°C for 3h, before being concentrated. The residue was dissolved in EtOAc and washed with sat. aq. NH4Cl. The aqueous layer was extracted with EtOAc and the combined organic layers werewashedwithNH4Cl(x2),dried(Na2SO4),filteredandconcentratedtoprovidemethyl6--thioureidohexanoate(2c,0.110mg,0.537mmol,78%)
References:
Manos-Turvey, Alexandra;Al-Ashtal, Hiba A.;Needham, Patrick G.;Hartline, Caroll B.;Prichard, Mark N.;Wipf, Peter;Brodsky, Jeffrey L. [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 20,p. 5087 - 5091] Location in patent:supporting information
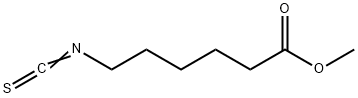
16424-87-6
8 suppliers
inquiry
![METHYL 6-[(AMINOCARBONOTHIOYL)AMINO]HEXANOATE](/CAS/GIF/98998-60-8.gif)
98998-60-8
11 suppliers
inquiry
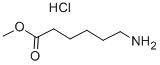
1926-80-3
104 suppliers
$5.00/250mg
![METHYL 6-[(AMINOCARBONOTHIOYL)AMINO]HEXANOATE](/CAS/GIF/98998-60-8.gif)
98998-60-8
11 suppliers
inquiry