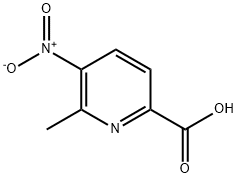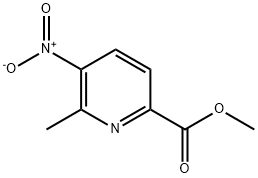
methyl 6-methyl-5-nitropicolinate synthesis
- Product Name:methyl 6-methyl-5-nitropicolinate
- CAS Number:64215-12-9
- Molecular formula:C8H8N2O4
- Molecular Weight:196.16
Yield:64215-12-9 86%
Reaction Conditions:
with thionyl chloride for 17 h;Reflux;
Steps:
14.1 Step 1. Synthesis of methyl 6-methyl-5-nitropyridine-2-carboxylate (C30).
A solution of 6-methyl-5-nitropyridine-2-carboxylic acid (4.0 g, 22 mmol) inmethanol (50 mL) was treated with thionyl chloride (8.22 mL, 113 mmol) and heated at reflux for 17 hours. After removal of solvent in vacuo, the residue was partitioned between ethyl acetate and saturated aqueous sodium bicarbonate solution. The organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure to afford the product. Yield: 3.7 g, 19 mmol, 86%. LCMS m/z 197.0 [M+H]. 1H NMR(400 MHz, DMSO-d6) ? 8.57 (d, J=8.4 Hz, 1H), 8.12 (d, J=8.4 Hz, 1H), 3.92 (5, 3H),2.77 (5, 3H).
References:
WO2016/9297,2016,A1 Location in patent:Page/Page column 107
22282-96-8
193 suppliers
$6.00/250mg

64215-12-9
18 suppliers
inquiry