
Methyl 7-fluoroisoquinoline-3-carboxylate synthesis
- Product Name:Methyl 7-fluoroisoquinoline-3-carboxylate
- CAS Number:480451-06-7
- Molecular formula:C11H8FNO2
- Molecular Weight:205.1851
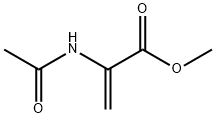
35356-70-8
161 suppliers
$19.00/250mg
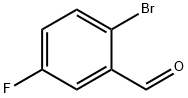
94569-84-3
301 suppliers
$16.00/5g

480451-06-7
8 suppliers
inquiry
Yield:480451-06-7 39%
Reaction Conditions:
with triethylamine;palladium diacetate in N,N-dimethyl-formamide at 110; for 4.5 h;Inert atmosphere;
Steps:
12A
Example 12A: methyl 7-fluoro-isoquinoline-3~carboxylate: A sealed tube was charged with 2-bromo-5-fluorobenzaidehyde (300 mg, 1.47 mmol), dry dimethylformamide (1.5 ml), triethylamine (0.7 ml, 5.02 mmol), methyl 2- acetamidoacryiate (273 mg, 1.91 mmol), fris-o-toiyi-phosphine (89 mg, 0.29 mmol) and palladium acetate (33 mg, 0.14 mmol). The solution was degassed with Argon for 10 min. and then warmed at 110°C for 4.5 hours. The mixture was cooled to room temperature and poured onto an aqueous solution of ammonium chloride. The mixture was extracted with ethyl acetate, washed with brine, dried over sodium sulfate and concentrated. The residue was purified by flash chromatography over silica gel (gradient cyclohexane/dichloromethane: 0-80%) to yield methyl 7-fluoro-isoqusnoline- 3-carboxylate (120 mg, 39%) as beige solid.1 H NMR: CDCl3 δ (ppm): 9.29 (s, 1 H), 8.60 (s, 1 H), 8.01 (dd, J = 9.0 Hz , J = 5.2 Hz, 1 H), 7.68 (dd, J = 8.4 Hz, J = 2.4 Hz, 1 H), 7.57 (td, J = 8.4 Hz, J = 2.4 Hz, 1 H), 4.06 (s, 3H).
References:
WO2011/51478,2011,A1 Location in patent:Page/Page column 57
![3-Isoquinolinecarboxylic acid, 7-fluoro-1,2,3,4-tetrahydro-2-[(4-methylphenyl)sulfonyl]-, methyl ester](/CAS/20210305/GIF/480451-05-6.gif)
480451-05-6
0 suppliers
inquiry

480451-06-7
8 suppliers
inquiry