
methyl ester -10-amino- Decanoic acid synthesis
- Product Name:methyl ester -10-amino- Decanoic acid
- CAS Number:106590-42-5
- Molecular formula:C11H23NO2
- Molecular Weight:201.31
Yield:106590-42-5 89%
Reaction Conditions:
with acetyl chloride at 0; for 3 h;Inert atmosphere;Reflux;
Steps:
4.1 5.1.4.1 10-Aminodecan-1-ol (16)
Freshly distilled acetyl chloride (1.70 mL, 23.90 mmol) was added dropwise to a stirred suspension of 10-aminodecanoic acid 12 (1.80 g, 9.61 mmol) in anhydrous MeOH (20 mL) under N2 atmosphere at 0 °C. Once the addition was complete, the mixture was heated under reflux for 3 h. After completion of the reaction, the solvent and excess acetyl chloride were distilled off under reduced pressure affording the corresponding methyl 10-aminodecanoate as a white solid (89%), which was used in the next reaction without further purification. Lithium aluminum hydride (1.65 g, 17.20 mmol) was added in small portions to a stirred suspension of the amino ester intermediate (1.73 g, 8.57 mmol) in anhydrous THF (100 mL) at 0 °C. The reaction mixture was heated under reflux for 48 h, then cooled to room temperature and poured into crushed ice. The aqueous layer was vigorously extracted with CH2Cl2 (6 x 80 mL) and the combined organic phases were washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure and the crude product was recrystallized to obtain pure 16 (0.92 g, 62%).
References:
Matera, Carlo;Pucci, Luca;Fiorentini, Chiara;Fucile, Sergio;Missale, Cristina;Grazioso, Giovanni;Clementi, Francesco;Zoli, Michele;De Amici, Marco;Gotti, Cecilia;Dallanoce, Clelia [European Journal of Medicinal Chemistry,2015,vol. 101,p. 367 - 383]
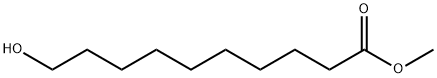
2640-94-0
24 suppliers
$259.00/10g

106590-42-5
1 suppliers
inquiry

186788-41-0
2 suppliers
inquiry

106590-42-5
1 suppliers
inquiry
![Decanoic acid, 10-[(methylsulfonyl)oxy]-, methyl ester](/CAS/20210305/GIF/874762-02-4.gif)
874762-02-4
0 suppliers
inquiry

106590-42-5
1 suppliers
inquiry

3927-60-4
51 suppliers
inquiry

106590-42-5
1 suppliers
inquiry

