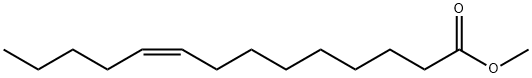
Methyl myristoleate synthesis
- Product Name:Methyl myristoleate
- CAS Number:56219-06-8
- Molecular formula:C15H28O2
- Molecular Weight:240.38
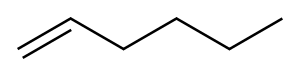
592-41-6
269 suppliers
$10.00/5g
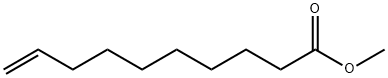
25601-41-6
48 suppliers
inquiry

56219-06-8
72 suppliers
$28.00/50mg
Yield:56219-06-8 25%
Reaction Conditions:
Stage #1:1-hexene;methyl 9-decenoate with triethylaluminum in toluene at 23 - 25; for 1 h;Cross Metathesis;
Stage #2: with tungsten (1R)-3,3’-dibromo-2’-[[(1,1-dimethylethyl)dimethylsilyl]oxyl-5,5’,6,6,7,7,8,8’-octahydro[1,1‘-binaphthalen]-2-olato-κO] [2,6-dichlorobenzenaminato(2-)-κN] 2,5-dimethyl-1H-pyrrol-1-yl[(2-methoxyphenyl)methylene] in toluene at 40 - 41; for 3 h;Catalytic behavior;Time;Temperature;Reagent/catalyst;
Steps:
6
Into a glass vessel equipped with an agitator, thermometer and reflux condenser, were charged 500 g of methyl dec-9-enoate (2.71 mol) and 480 g of hex-1-ene (5.70 mol). To the thoroughly homogenized feedstocks a solution of triethylaluminum in toluene (3.82 g, 0.0335 mol, 0.389 mol %) was added in one portion. After agitating at 500 rpm for an hour at 23-25° C., the temperature of the feedstock was raised to 40-41° C. 0.121 g (0.00128 mol %, 123 ppmwt) of tungsten, [(1R)-3,3′-dibromo-2′-[[(1,1-dimethylethyl)dimethylsilylloxy]-5,5′, 6,6′,7,7′,8,8′-octahydro[1,1′-binaphthalen]-2-olato-κO][2,6-dichlorobenzenaminato(2-)-κN](2,5-dimethyl-1H-pyrrol-1-yl)[(2-methoxyphenyl)methylene]-, (T-4)-(CAS 1817807-15-0) was added in four portions to control the rate of ethylene generation and the reaction was allowed to proceed for three hours. After that time GC-FID analysis showed the reaction proceed in 57% Methyl Dec-9-enoate Conversion and 96% Methyl (Z)-tetradec-9-enoate Selectivity. To the cooled (25-30° C.) reaction mixture was added 10 mL of methanol (H2O=0.035-0.038 w %). The mixture was stirred at ambient temperature for 15-20 minutes. The aliquot was then removed from the reactor and filtered through a plug comprising a lower 0.5 cm layer of diatomaceous earth and an upper 1.0 cm layer of silica gel. The filter cake was washed with 7×100mL MTBE. The volume of the colorless and clear filtrate was concentrated under reduced pressure in a 45° C. water bath at a pressure of 40 mbar to obtain the crude product as a colorless liquid. The crude material was vacuum distilled (0.2-1 mbar) using a short path distillation apparatus and 166 g (25% overall yield) of methyl (Z)-tetradec-9-enoate was collected at a head temperature of 95-97° C. and pressure of 0.4 mbar.
References:
PROVIVI, INC.;WAMPLER, KEITH M.;MEINHOLD, PETER;COELHO, PEDRO;BUI, VU;ONDI, LEVENTE;MEHDI, HASAN US2017/137365, 2017, A1 Location in patent:Paragraph 0391

1931-63-1
47 suppliers
inquiry
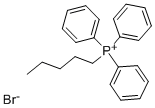
21406-61-1
175 suppliers
$6.00/5g

56219-06-8
72 suppliers
$28.00/50mg

1931-63-1
47 suppliers
inquiry

21406-61-1
175 suppliers
$6.00/5g

72025-18-4
30 suppliers
$158.00/100mg

56219-06-8
72 suppliers
$28.00/50mg
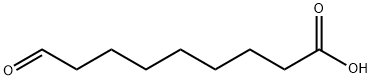
2553-17-5
34 suppliers
$35.00/5mg

56219-06-8
72 suppliers
$28.00/50mg
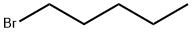
110-53-2
414 suppliers
$12.50/5g

56219-06-8
72 suppliers
$28.00/50mg