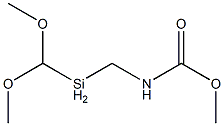
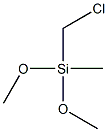
METHYL [N-(DIMETHOXYMETHYL)SILYLMETHYL]CARBAMATE synthesis
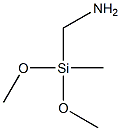
- Product Name:METHYL [N-(DIMETHOXYMETHYL)SILYLMETHYL]CARBAMATE
- CAS Number:23432-65-7
- Molecular formula:C6H15NO4Si
- Molecular Weight:193.27
Yield:23432-65-7 95%
Reaction Conditions:
with sodium methylate in methanol at 40 - 80; for 3 h;
Steps:
3a Process for preparing N-(methyldimethoxysilylmethyl) O-methyl carbamate
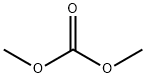
A mixture of 360.3 g (3.97 mol) of dimethyl carbonate and 2.99 g of a 30% strength by weight solution of sodium methoxide in methanol (corresponding to 0.90 g of pure sodium methoxide) is placed in a 2 l four-neck flask provided with dropping funnel, Liebig condenser, precision glass stirrer and thermometer and heated to 55° C. At this temperature, 386.0 g (2.83 mol) of aminomethylmethyldimethoxysilane are introduced over a period of 60 minutes. In order to maintain the temperature, gentle cooling is necessary. (0102) The mixture is subsequently stirred at 40° C. for another 1 hour and then heated to 80° C. This temperature is maintained for a further 1 hour. (0103) Finally, 1.30 g of sulfuric acid (98% strength) are added. Only very slight clouding of the reaction mixture occurs here. A drop taken from the reaction mixture is placed on a previously moistened pH paper. The reaction mixture displays a pH of from 6 to 7. (0104) The low boilers are removed by distillation from the neutralized reaction mixture. For this purpose, the pressure is reduced stepwise to 1 mbar, while the temperature at the bottom firstly remains at 80° C. and is finally increased once more to 110° C. The distillation is concluded as soon as no more distillate goes over. Analysis of the distillate by means of GC and/or 1H-NMR shows that the distillate consists virtually exclusively (i.e. to an extent of more than 99%) of the methanol liberated, the dimethyl carbonate used in excess and small amounts of methyltrimethoxysilane. (0105) A light-yellow product is obtained in a purity of 96.8%. The yield is high (>95%) based on the aminosilane used. Filtration to separate off the sodium sulfate formed is not necessary.
References:
US2017/320896,2017,A1 Location in patent:Paragraph 0101-0105

67-56-1
737 suppliers
$9.00/25ml
590-28-3
243 suppliers
$10.00/5g
2212-11-5
82 suppliers
$149.00/5g
![METHYL [N-(DIMETHOXYMETHYL)SILYLMETHYL]CARBAMATE](/CAS/GIF/23432-65-7.gif)
23432-65-7
24 suppliers
inquiry