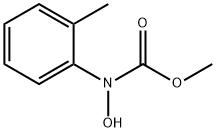
Methyl N-Methoxy-2-methylphenylcarbamate synthesis
- Product Name:Methyl N-Methoxy-2-methylphenylcarbamate
- CAS Number:151827-82-6
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
Yield:151827-82-6 96.2%
Reaction Conditions:
with tert-butyl methyl ether;tetrabutyl-ammonium chloride;sodium hydroxide in water;chlorobenzene;Autoclave;Alkaline conditions;Reagent/catalyst;Solvent;
Steps:
3 example 3
1) The pyraclostrobin intermediate and a phase transfer catalyst, a solvent, and an alkali solution are added to the autoclave, and the atmosphere in the autoclave is replaced with methyl chloride gas. The phase transfer catalyst used benzyltriethylammonium chloride. The solvent is selected from water-insoluble organic solvents such as chlorobenzene or dichlorobenzene selected from (halo)aromatic hydrocarbons. The alkaline solution was a 40% aqueous sodium hydroxide solution.The mass ratio of the pyraclostrobin intermediate to the selected phase transfer catalyst and the solvent was controlled to 1:0.02:5; the equivalent ratio to the alkali and monochloromethane gas was controlled to 1:1.4:2.2) The temperature is controlled at 80 °C, and the pressure of chlorine gas is maintained at 0.8 MPa;3) After the reaction for 6 hours, samples were taken from the sample reactor. When Pyraclostrobin intermediate was detected by HPLC and the content was less than 0.5%, the reaction was stopped. After cooling and cooling, the pyraclostrobin was obtained after phase separation, solvent removal, and recrystallization. The methylation product of the intermediate, the HPLC external standard content was 98.8%, and the yield was 96.2%.
References:
CN107973751,2018,A Location in patent:Paragraph 0041-0047
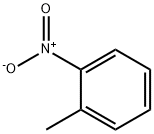
88-72-2
249 suppliers
$15.00/5g

151827-82-6
6 suppliers
inquiry

611-22-3
5 suppliers
inquiry

151827-82-6
6 suppliers
inquiry