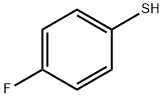
methyl p-fluorobenzenesulphonate synthesis
- Product Name:methyl p-fluorobenzenesulphonate
- CAS Number:565-45-7
- Molecular formula:C7H7FO3S
- Molecular Weight:190.19
Yield:565-45-7 45%
Reaction Conditions:
with oxygen;9-(2-mesityl)-10-methylacridinium perchlorate at 20; for 72 h;Sealed tube;Schlenk technique;Irradiation;Green chemistry;
Steps:
Alkyl Benzenesulfonates 3; General Procedure
General procedure: An oven-dried Schlenk tube equipped with a magnetic stirrerbar was charged with Acr+Mes ClO4- (5.0 mol%), capped with aseptum, and evacuated. A balloon filled with O2 was connectedto the Schlenk tube through the side arm, and thiophenol 1(1.0mmol) and alcohol 2 (3.0 mL) were successively injected intothe reaction tube. The mixture was irradiated with light fromblue LEDs (3.0 W) and vigorously stirred at r.t. for 18-72 h (seeScheme 2). When the reaction was complete (TLC), the productwas purified by flash column chromatography (silica gel, PE-EtOAc).
References:
Singh, Atul K.;Yi, Hong;Zhang, Guoting;Bian, Changliang;Pei, Pengkun;Lei, Aiwen [Synlett,2017,vol. 28,# 13,p. 1558 - 1563] Location in patent:supporting information