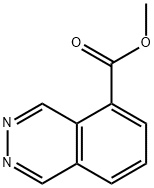
Methyl phthalazine-5-carboxylate synthesis
- Product Name:Methyl phthalazine-5-carboxylate
- CAS Number:1104070-92-9
- Molecular formula:C10H8N2O2
- Molecular Weight:188.18

67-56-1
739 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

103119-78-4
40 suppliers
$175.00/50mg

1104070-92-9
10 suppliers
inquiry
Yield:1104070-92-9 32%
Reaction Conditions:
with potassium acetate;triphenylphosphine;palladium diacetate in N,N-dimethyl-formamide at 100; under 2068.65 Torr; for 15 h;
Steps:
28
Methyl phthalazine-5-carboxylate. To a 140 mL pressure flask was added 5-bromophthalazine (3.5 g, 17 mmol), palladium (II) acetate (0.98 g, 4.4 mmol), triphenylphosphine (1.3 g, 5.0 mmol), potassium acetate (2.1 g, 21 mmol), MeOH (20 mL) and DMF (20 mL). The flask was sealed and purged with CO (3 x). The flask was charged with CO to 40 PSI and stirred at 100°C 15 hours. The suspension was filtered through Celite, the cake was washed with MeOH, and the filtrate was concentrated. The residue was taken up in DCM and washed with saturated sodium bicarbonate (2 x), saturated sodium chloride (2 x) and water (2 x). The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo. The residue was adsorbed on to a plug of silica gel and purified by chromatography through a Redi-Sep pre-packed silica gel column, eluting with a gradient of EtOAc in hexane to provide methyl phthalazine-5- carboxylate (1.0 g, 32 %). LCMS (M+H) 189.2 calc. for C10H9N2O2 189.1, 1H NMR (400 MHz, CDCl3): δ ppm 4.05 (s, 3H) 8.06 (d, J=8.41 Hz, 1H) 8.51-8.56 (m, 1H) 8.71 (d, 1H) 9.64 (s, 2H).
References:
WO2009/11871,2009,A2 Location in patent:Page/Page column 153; 154