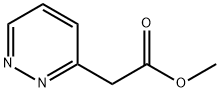
Methyl pyridazin-3-yl-acetate synthesis
- Product Name:Methyl pyridazin-3-yl-acetate
- CAS Number:37444-32-9
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
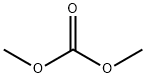
616-38-6
660 suppliers
$5.00/5G

37444-32-9
28 suppliers
$331.00/100mg
Yield:37444-32-9 11%
Reaction Conditions:
Stage #1: 3-methylpyridazinewith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -78; for 1.00033 h;
Stage #2: carbonic acid dimethyl ester in tetrahydrofuran at -78 - 25; for 48 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran;
Steps:
35.a
a). To a solution of diisopropylamine (19 ml, 130 mmol, 1.3 equiv) in dry THF (100 mL) at 0° C. under a N2, was added n-butyllithium (81 ml, 130 mmol, 1.3 equiv) slowly. The mixture was stirred at 0° C. for 30 min and then cooled to -78° C. A solution of 3-methylpyridazine (9 ml, 100 mmol, 1.0 equiv) in THF (50 mL) was added dropwise over a period of 1:5 min. The resulting mixture was stirred at -78° C. for 1 h, and a solution of dimethyl carbonate (17 ml, 200 mmol, 2 equiv) in THF (50 mL) was added dropwise. The mixture was allowed to warm slowly to 25° C. and stirred for 2 days. Sat.NH4Cl was added and the resulting solution was extracted with 10% MeOH/DCM. The combined organics were washed with water and brine, dried over Na2SO4 and concentrated. The residue was purified by flash chromatography (2.5% MeOH/DCM) to give 1.6 g of methyl 2-(pyridazin-3-yl)acetate as a brown liquid (11 mmol, 11%).
References:
US2007/299080,2007,A1 Location in patent:Page/Page column 82-83