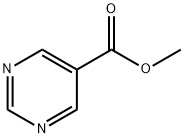
METHYL PYRIMIDINE-5-CARBOXYLATE synthesis
- Product Name:METHYL PYRIMIDINE-5-CARBOXYLATE
- CAS Number:34253-01-5
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
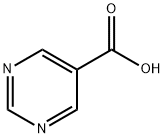
4595-61-3
234 suppliers
$6.00/1g

34253-01-5
102 suppliers
$39.00/250 mg
Yield:-
Reaction Conditions:
with potassium hydroxide;acetic acid in tetrahydrofuran;ethanol;water
Steps:
1.1 5-Methoxycarbonylpyrimidine
1) Crude pyrimidine-5-carboxylic acid (1.24 g, 10 mmol) was dissolved in 100 ml THF (dry), 3 ml water, and cooled to 0° C. in the reaction vessel of a Mini-Diazald apparatus (Aldrich Chemical). Diazald (3 g, 14 mmol) dissolved in ether (27.5 ml) was added dropwise over 15 minutes to KOH (3 g) in ethanol/water (11 ml) at 65° C. to generate diazomethane. Ether (20 ml) was added dropwise, after the Diazald solution, to co-distill the remaining diazomethane into the reaction flask. The reaction was allowed to stir 3 hours until nitrogen evolution had stopped, and excess diazomethane was then destroyed by adding acetic acid. The solvents were evaporated in vacuo,. the residue taken up in 50 ml water, basified to pH 9 (NaCO3), and extracted with chloroform (3*100 ml). After drying (MgSO4), filtering, and evaporating the solvent in vacuo, 1.26 g (91%) crude crystals were obtained. Recrystallization from chloroform/hexane gave 606 mg (44%) light yellow crystals mp 81.6°-86.6° C. Microanalysis calc.: C 52.17, H 4.38, N 20.29; found: C 51.95, H 4.31, N 19.96. 400 MHz nmr indicated product.
References:
The University of Toledo US5175166, 1992, A
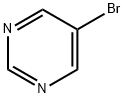
4595-59-9
461 suppliers
$6.00/1g

67-56-1
739 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

34253-01-5
102 suppliers
$39.00/250 mg