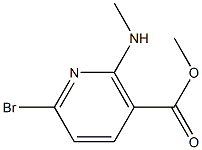
Methyl6-bromo-2-(methylamino)nicotinate synthesis
- Product Name:Methyl6-bromo-2-(methylamino)nicotinate
- CAS Number:1034131-15-1
- Molecular formula:C8H9BrN2O2
- Molecular Weight:245.0733
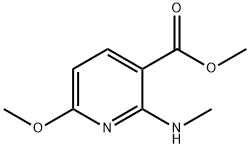
503000-88-2
5 suppliers
inquiry

1034131-15-1
3 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: methyl 6-methoxy-2-methylaminopyridine-3-carboxylatewith phosphorus(V) oxybromide;phosphoric acid;Pyridine hydrobromide in benzene;Heating / reflux;
Stage #2: with sodium carbonate in water;benzene; pH=7 - 8;
Steps:
11.d
(d) Methyl 6-bromo-2-(methylamino)nicotinate. A mixture of methyl 6-methoxy-2-(methylamino)nicotinate (20 g, 100 mmol), POBr3 (58.6 g, 204.4 mmol), H3PO4 (1.0 g, 10.2 mmol) and C5H5N-HBr (1.6 g, 10.0 mmol) in benzene (200 mL) was heated at reflux overnight. The reaction mixture was left to reach room temperature, poured into ice-water, basified to pH = 7-8 with sodium carbonate, and extracted with DCM. The organic layer was washed with water and brine, dried over anhydrous Na2SO4, and filtered. The filtrate was evaporated under reduced pressure, and the residue purified by silica gel chromatography to give the title compound.
References:
WO2008/76425,2008,A1 Location in patent:Page/Page column 43
38496-18-3
365 suppliers
$6.00/1g

1034131-15-1
3 suppliers
inquiry

503000-87-1
84 suppliers
$37.00/250mg

1034131-15-1
3 suppliers
inquiry