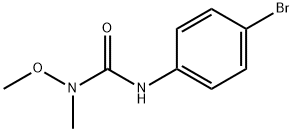
Metobromuron synthesis
- Product Name:Metobromuron
- CAS Number:3060-89-7
- Molecular formula:C9H11BrN2O2
- Molecular Weight:259.1
2493-02-9
82 suppliers
$39.50/5g
77-78-1
289 suppliers
$19.69/1ml

3060-89-7
67 suppliers
$38.10/100mg
Yield:-
Reaction Conditions:
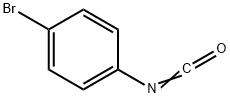
Stage #1:4-bromophenyl isocyanate with hydroxylamine hydrochloride;sodium hydroxide in water;toluene; pH=7 - 8 at 12;
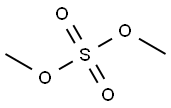
Stage #2:dimethyl sulfate with sodium hydroxide in water; pH=8 - 10 at 20 - 25;Reagent/catalyst;Solvent;Temperature;
Steps:
1.1 Example 1,
(1) Add 10.4 kg of hydroxylamine hydrochloride to 86 kg of water.Then add 32% sodium hydroxide solution,The pH of the reaction solution is 7-8.Then add 100 kg of toluene,19.8 kg of p-bromophenyl isocyanate was added dropwise at 12°C.The insulation reaction is performed after the dripping is completed.The weight content of p-bromophenyl isocyanate in HPLC was less than 0.5%, and 22.4 kg of solid was filtered;
(2) Adding the solid obtained above to 110 kg of water,Adding a 32% NaOH solution to adjust the pH of the reaction solution is 8-10.Add 15 kg of dimethyl sulfate dropwise at 20-25 °C.At the same time plus 32% sodium hydroxide solution to control the pH of the reaction solution is 8 to 10,Dimethyl sulphate dripping after the insulation reaction,The HPLC-controlled 3-(4-bromophenyl)-1-hydroxyurea weight content is less than 0.5%.Filter, filter cake, filter cake washed with 10 kg of water,Then the product was blown with air at 60-70°C to obtain the final product 3-(4-bromophenyl)-1-methoxy-1-methylurea (23.8 kg).The two-step total yield was 91.9% and the purity was 99.1%.
References:
Changzhou Woteng Chemical Technology Co., Ltd.;Li Honggong;Wei Xinghong;Wang Mingfeng;Liu Rong;Sun Kezhou CN106831492, 2017, A Location in patent:Paragraph 0009; 0010; 0011

1576-17-6
3 suppliers
$144.00/250mg

3060-89-7
67 suppliers
$38.10/100mg