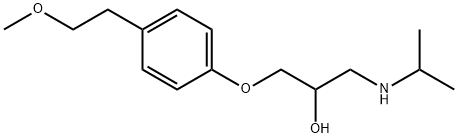
Metoprolol synthesis
- Product Name:Metoprolol
- CAS Number:51384-51-1
- Molecular formula:C15H25NO3
- Molecular Weight:267.36
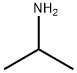
75-31-0
411 suppliers
$14.00/25mL
![[[p-(2-methoxyethyl)phenoxy]methyl]oxirane](/CAS/GIF/56718-70-8.gif)
56718-70-8
129 suppliers
$80.00/1 g

51384-51-1
83 suppliers
$45.00/100mg
Yield:51384-51-1 95%
Reaction Conditions:
Stage #1:isopropylamine;2-[4-(2'-methoxyethyl)phenoxymethyl]oxirane in N,N-dimethyl-formamide at 60; for 12 h;Sealed tube;
Stage #2: with water in N,N-dimethyl-formamide at 60; for 12 h;Sealed tube;regioselective reaction;Solvent;Temperature;
Steps:
1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol (Metoprolol)
2-{[4-(2-Methoxyethyl)phenoxy]methyl}oxirane (20 g, 93.1 mmol) and propan-2-amine (8.34 g, 139.7 mmol) were combined in DMF and heated to 60 °C in a pressure vessel for 12 h, after which the vessel was allowed to cool to r.t. before adding deionized H2O (32.28 mL, 50 equiv) in one portion. The flask was resealed and allowed to react for an additional 12 h at 60 °C. Solvent and excess amine were removed on a rotary evaporator (35 °C/22.5) to afford 24.5 g (95%) of metoprolol freebase as a white semi-solid in >95% purity (1H NMR); Rf = 0.28 (1:9 MeOH/CHCl3). IR (thin film): 3299, 3034, 2966, 1613 cm-1. 1H NMR (400 MHz, CDCl3): δ = 7.03 (d, J = 7.1 Hz, 2 H), 6.77 (d, J = 7.1 Hz, 2 H), 4.00 (ddt, J = 7.5, 7.3, 6.8 Hz, 1 H), 3.83-5.09 (m, 2 H), 3.49 (t, J = 7.5 Hz, 2 H), 3.27 (s, 3 H), 3.23 (br s, 1 H), 3.78-3.72 (m, 4 H), 2.71 (dd, J = 6.8, 6.6 Hz, 1 H), 1.03 (d, J = 6.9 Hz, 6 H). 13C NMR (100 MHz, CDCl3): δ = 157.0, 131.0, 129.5, 114.2, 73.6, 70.6, 68.1, 58.4, 59.5, 48.7, 35.1, 22.7, 22.6. ESI-MS: m/z (%) = (pos) 268.2 ([M + H]+, 100); (neg) 266.2 ([M - H]-, 100).
References:
Lizza, Joseph R.;Moura-Letts, Gustavo [Synthesis,2017,vol. 49,# 6,art. no. SS-2016-M0690-OP,p. 1231 - 1242] Location in patent:supporting information
56718-71-9
412 suppliers
$6.00/5g

51384-51-1
83 suppliers
$45.00/100mg

75-31-0
411 suppliers
$14.00/25mL
![[[p-(2-methoxyethyl)phenoxy]methyl]oxirane](/CAS/GIF/56718-70-8.gif)
56718-70-8
129 suppliers
$80.00/1 g

51384-51-1
83 suppliers
$45.00/100mg
![1,1[(1-Methylethyl)imino]bis[3-[4-(2-methoxyethyl)phenoxy]-2-propanol_x000b_(Mixture of diastereomers)](/CAS/GIF/154784-36-8.gif)
154784-36-8
64 suppliers
$160.00/5 mg