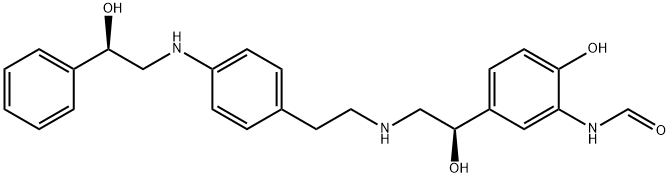
Milveterol synthesis
- Product Name:Milveterol
- CAS Number:652990-07-3
- Molecular formula:C25H29N3O4
- Molecular Weight:435.52
![Formamide, N-[5-[(1R)-1-hydroxy-2-[[2-[4-[[(2R)-2-hydroxy-2-phenylethyl]amino]phenyl]ethyl]amino]ethyl]-2-(phenylmethoxy)phenyl]-](/CAS/20210305/GIF/652990-10-8.gif)
652990-10-8
0 suppliers
inquiry

652990-07-3
4 suppliers
inquiry
Yield:652990-07-3 91%
Reaction Conditions:
Stage #1: N-{2-[4-((R)-2-hydroxy-2-phenylethylamino)phenyl]ethyl}-(R)-2-hydroxy-2-(3-formamido-4-benzyloxyphenyl)ethylaminewith darco G-60 in ethanol at 50; for 0.333333 h;
Stage #2: with hydrogen;palladium 10% on activated carbon in ethanol; under 1551.49 Torr; for 10 h;
Steps:
5 EXAMPLE 5; Synthesis of N-{2-[4-((R)-2-hydroxy-2-phenylethylamino)phenyl]ethyl}-(R)-2-hydroxy-2-(3-formamido-4-hydroxyphenyl)ethylamine (1)
Intermediate 6 (2.5 g, 4.8 mmol) was dissolved in 8.0 mL of ethanol and treated with activated charcoal, Darco G-60 (1.25 g). The suspension was stirred at 50 C. for 20 min and then filtered to remove the Darco. To the filtrate was added 10% palladium on activated carbon (250 mg) and the suspension placed on a Parr shaker. The reaction was shaken for 10 h under 30 psi hydrogen gas. The reaction was filtered through celite and concentrated under vacuum to afford compound 1 as a brown gelatinous solid (1.9 g, 4.3 mmol, 91%). 1H NMR (300 MHz, DMSO-d6) ?2.40-2.68 (m, 6H), 2.92-3.18 (m, 2H), 4.35-4.45 (m, 1H), 4.60-4.69 (m, 1H), 5.22-5.30 (m, 1H), 6.82 (s, 1H),6.85 (s, 1H), 6.68-6.86 (m, 4H), 7.12-7.36 (m, 5H), 7.95 (d, 1H, J=1.4 Hz), 8.19 (s, 1H), 9.49 (br s, 1H). m/z: [M+H+] calcd for C25H29N3O4436.2; found 436.4.
References:
US2004/248985,2004,A1 Location in patent:Page 11
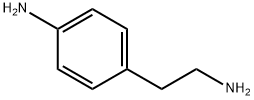
13472-00-9
256 suppliers
$18.00/1g

652990-07-3
4 suppliers
inquiry
![Benzeneethanamine, N-(phenylmethylene)-4-[(phenylmethylene)amino]-](/CAS/20210305/GIF/344466-46-2.gif)
344466-46-2
0 suppliers
inquiry

652990-07-3
4 suppliers
inquiry
![Benzeneethanamine, N-(phenylmethyl)-4-[(phenylmethyl)amino]-](/CAS/20210305/GIF/344466-47-3.gif)
344466-47-3
0 suppliers
inquiry

652990-07-3
4 suppliers
inquiry
![Benzenemethanol, α-[[(phenylmethyl)[4-[2-[(phenylmethyl)amino]ethyl]phenyl]amino]methyl]-, (αR)-](/CAS/20210305/GIF/1355183-83-3.gif)
1355183-83-3
0 suppliers
inquiry

652990-07-3
4 suppliers
inquiry