
MJXIPHMGYJXKLJ-HUIMPEKJSA-N synthesis
- Product Name:MJXIPHMGYJXKLJ-HUIMPEKJSA-N
- CAS Number:161511-08-6
- Molecular formula:C24H38N5O11P
- Molecular Weight:603.5592
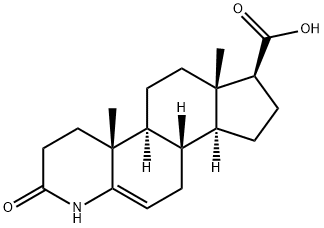
103335-54-2
32 suppliers
inquiry

161511-08-6
0 suppliers
inquiry
Yield:161511-08-6 95.2%
Reaction Conditions:
with 3% Pd/C;hydrogen;sodium hydroxide in water at 38; under 225.023 Torr; for 12 h;Inert atmosphere;Large scale;Pressure;Reagent/catalyst;Temperature;
Steps:
1
A 1500 L enamel closed reaction kettle equipped with a thermometer and a stirrer was charged with 1000 L of water and stirred and 13.5 kg of sodium hydroxide was added. After dissolving, 3-carbonyl-4-aza-5-androstene -17β-carboxylic acid 100kg, dissolved, add 3% palladium carbon 10kg.The jacket of the reactor was heated to 38 ° C (controlled at 38 ° C, the actual measured value fluctuates by ± 2 ° C) by exchanging hot water with nitrogen gas and hydrogen gas for three times, hydrogen gas was supplied to 0.03 MPa, intermittent replenishment of hydrogen Reaction 12h, TLC plate is not color, the discharge of hydrogen, with nitrogen replacement.The reaction mixture was filtered, washed with tap water, drained and recovered by palladium carbon filter cake.The filtrate was adjusted to pH = 4 with concentrated hydrochloric acid, washed with water and tap water until the eluate was neutral and dried to give crude 3-oxo-4-azaandrosta-17β-carboxylic acid I. HPLC: purity 98.5533%; Isomer 1.1487%, unknown impurity 0.2064% and raw material II 0.0915%
References:
CN104311626,2016,B Location in patent:Paragraph 0034 - 0037
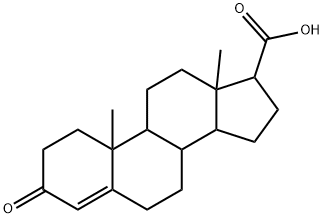
302-97-6
177 suppliers
$29.00/5mg

161511-08-6
0 suppliers
inquiry