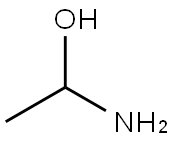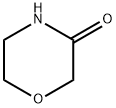
morpholin-3-one synthesis
- Product Name:morpholin-3-one
- CAS Number:109-11-5
- Molecular formula:C4H7NO2
- Molecular Weight:101.1
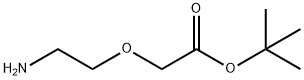
1155811-37-2
27 suppliers
inquiry

109-11-5
272 suppliers
$5.00/1g
Yield:109-11-5 94.6%
Reaction Conditions:
with trifluoroacetic acid in toluene for 3 h;Reflux;Large scale;
Steps:
1.4; 2.4; 3.4; 4.4 Step 4: Preparation of 3-morpholinone I
To a 50 L reactor, 4.91 kg of a colorless oil, 24.6 L of toluene and 637.5 g of trifluoroacetic acid obtained in the above step 3 were added, and the mixture was heated to reflux for 3 h.TLC (methanol: dichloromethane = 1:9, iodine coloration) showed that the deprotection II reaction was complete, the heating was removed, and after cooling to room temperature,The reaction solution was washed with 13 L×2 times saturated sodium bicarbonate solution, and after liquid separation,The organic layer was washed successively with 13 L of water and 13 L of saturated brine.The washed organic layer was evaporated to dryness to give a white powdery solid.The white powdery solid was recrystallized from 8.5 L of methyl tert-butyl ether, and dried to give 2.68 kg of white needle crystals.The yield was 94.6%.Spectroscopic analysis confirmed 3-morpholinone I.
References:
Zhejiang Jingxin Pharmaceutical Co., Ltd.;Peng Chunyong;Wang Zhi;Zhu Jianrong;Zhang Qin CN109422703, 2019, A Location in patent:Paragraph 0034; 0042; 0049-0050; 0052; 0059-0060; 0062-0080
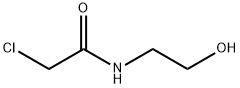
6320-16-7
9 suppliers
inquiry

109-11-5
272 suppliers
$5.00/1g
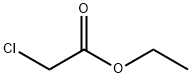
105-39-5
413 suppliers
$10.00/5g

109-11-5
272 suppliers
$5.00/1g