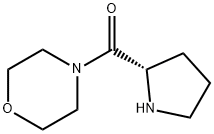
MORPHOLIN-4-YL-(S)-PYRROLIDIN-2-YL-METHANONE synthesis
- Product Name:MORPHOLIN-4-YL-(S)-PYRROLIDIN-2-YL-METHANONE
- CAS Number:73094-26-5
- Molecular formula:C9H16N2O2
- Molecular Weight:184.24
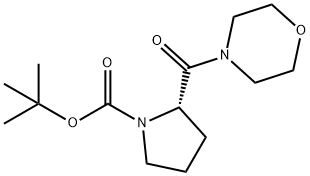
537040-24-7
0 suppliers
inquiry

73094-26-5
13 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 2 h;
Steps:
General procedure: TFA (8 mL) was added to the solution of (S)-tert-butyl 2-(piperidine-1-carbonyl)pyrrolidine-1-carboxylate 3 (0.82 g, 2.9 mmol), in DCM (2 mL) and stirred for 2 h at room temperature. The excess reagent and solvent were removed under reduced pressure and left under vacuum overnight. Then the residue was dissolved in DCM (15 ml). and cooled to 0 C. TEA (1.9 g, 18.85 mmol) was added followed by dropwise addition of 5-bromopentanoyl chloride (0.74 g, 3.73 mmol,), dissolved in DCM (8 ml). The stirring was continued for 12 h. Solvent was evaporated under reduced pressure and the N-acylated product 13 was purified by column chromatography using silica gel and a mixture of DCM/MeOH = 9/0.7 (v/v) as an eluting system to yield 0.74 g (74%) of product 13 as colorless oil.
References:
Canale, Vittorio;Kurczab, Rafa?;Partyka, Anna;Sata?a, Grzegorz;Witek, Jagna;Jastrz?bska-Wi?sek, Magdalena;Paw?owski, Maciej;Bojarski, Andrzej J.;Weso?owska, Anna;Zajdel, Pawe? [European Journal of Medicinal Chemistry,2015,vol. 92,p. 202 - 211] Location in patent:supporting information
66165-42-2
0 suppliers
inquiry

73094-26-5
13 suppliers
$45.00/50mg
1148-11-4
447 suppliers
$6.00/10g

73094-26-5
13 suppliers
$45.00/50mg
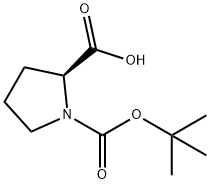
15761-39-4
602 suppliers
$5.00/10g

73094-26-5
13 suppliers
$45.00/50mg