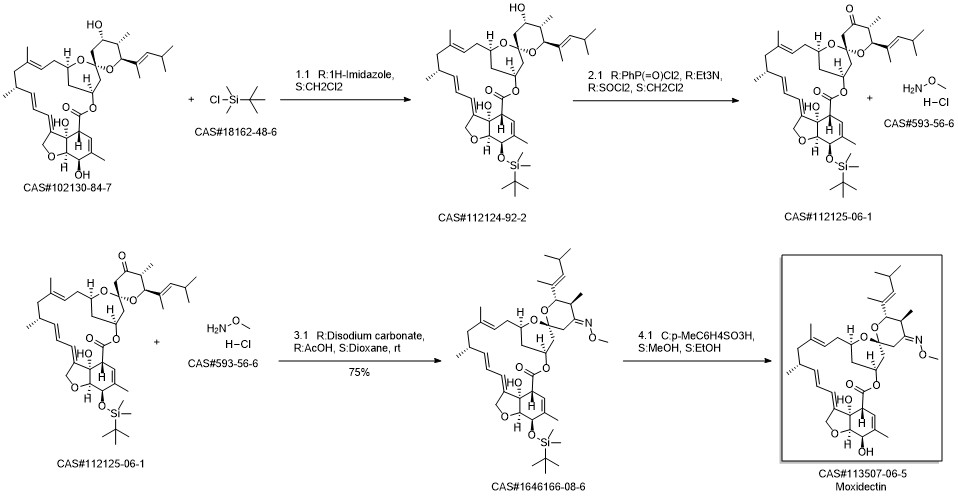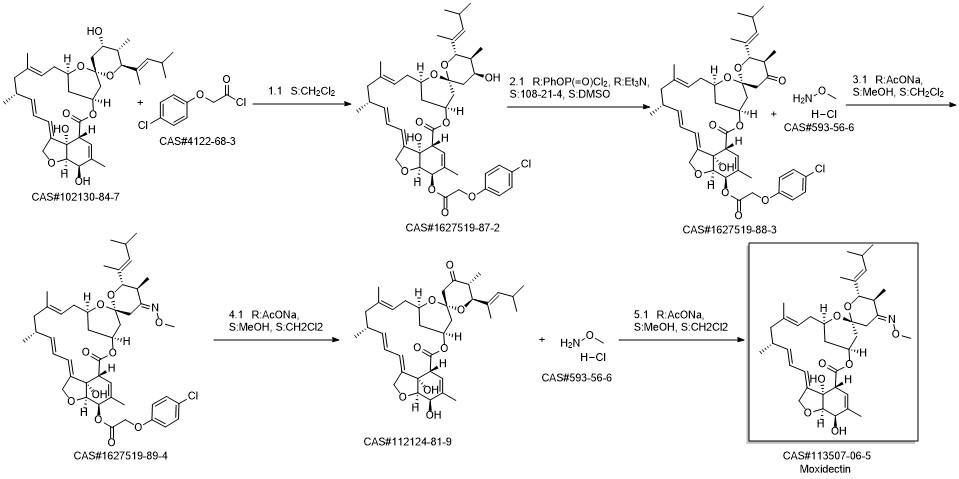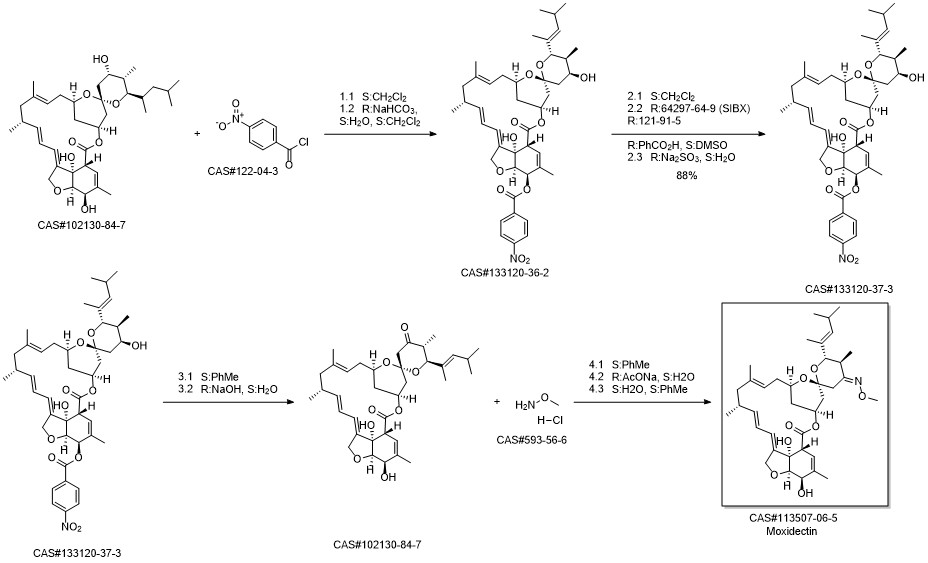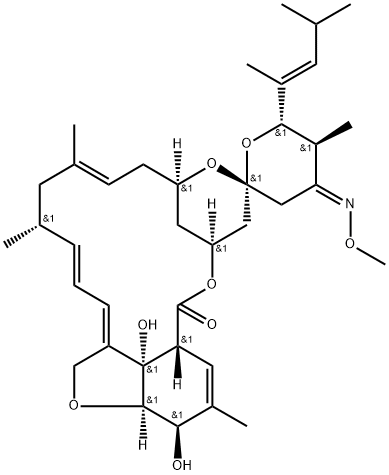
Moxidectin synthesis
- Product Name:Moxidectin
- CAS Number:113507-06-5
- Molecular formula:C37 H53 N O8
- Molecular Weight:639.82
Reference: Beddall, Nicola E.; Howes, Peter D.; Ramsay, Michael V. J.; Roberts, Stanley M.; Slawin, Alexandra M. Z.; Sutherland, Derek R.; Tiley, Edward P.; Williams, David J. Chemical transformations of S541 factors (A)-(D): preparation and reactions of the 23-ketones. Tetrahedron Letters. Volume 29. Issue 21. Pages 2595-8. Journal. (1988).

112124-81-9
20 suppliers
inquiry

113507-06-5
351 suppliers
$32.00/5mg
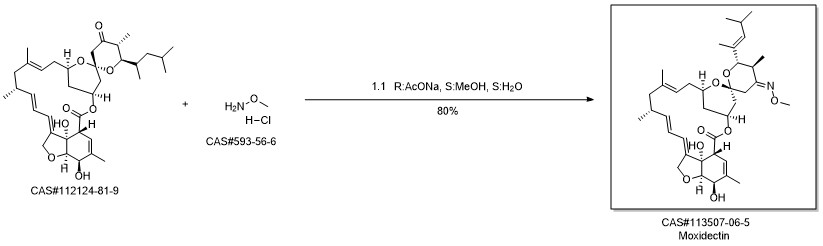
Yield:113507-06-5 88.6%
Reaction Conditions:
with N-methoxylamine hydrochloride;sodium acetate in methanol at -10 - 0; for 8 h;
Steps:
1.D; 8-9 D. Preparation of Moxectine (I)
Add 23-oxonimoxine (IV) (611g, 1.0mol), anhydrous sodium acetate (395g) and methanol (3600g) into the reactor, stir well and cool to -10 to 0, then stir again Add methoxyamine hydrochloride (183.7g, 2.2mol) in batches. During the addition, control the temperature of the mixture to not exceed 0°C. After the addition is complete, keep stirring and react under 0°C for 8 hours. After the reaction, add dropwise Adjust the pH value of the mixture to neutral with 5% sodium bicarbonate aqueous solution, and then add dichloromethane (6100g) to it for extraction. After separation, the organic phase is dried with anhydrous sodium sulfate. After drying, the desiccant is removed by filtration and the filtrate is concentrated. Obtained oil, recrystallized from methanol to obtain moxectine (I) fine product, 566.8g as a light yellow powder, the yield is about 88.6%,
References:
CN111592553,2020,A Location in patent:Paragraph 0046; 0053-0054; 0073-0078

593-56-6
596 suppliers
$8.00/100dtn(0.1g)

112124-81-9
20 suppliers
inquiry

113507-06-5
351 suppliers
$32.00/5mg
102130-84-7
69 suppliers
$265.00/1mg

113507-06-5
351 suppliers
$32.00/5mg
![Milbemycin B, 5-O-acetyl-5-O-demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-oxo-, [6R,25S(E)]- (9CI)](/CAS/20211123/GIF/113462-09-2.gif)
113462-09-2
0 suppliers
inquiry

113507-06-5
351 suppliers
$32.00/5mg
108702-57-4
0 suppliers
inquiry

113507-06-5
351 suppliers
$32.00/5mg