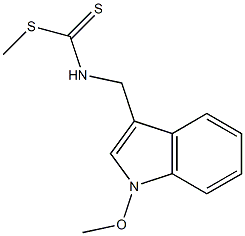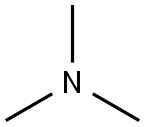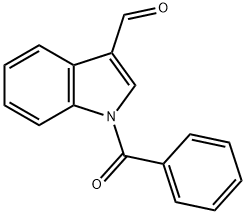
N-1-benzoylindole-3-carboxaldehyde synthesis
- Product Name:N-1-benzoylindole-3-carboxaldehyde
- CAS Number:27092-42-8
- Molecular formula:C16H11NO2
- Molecular Weight:249.26
Yield:27092-42-8 98%
Reaction Conditions:
Stage #1: 1-methoxybrassininwith triethylamine in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: benzoyl chloride in tetrahydrofuran at 0; for 0.75 h;
Steps:
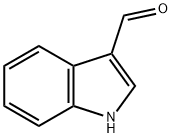
To a solution of indole-3-carboxaldehyde (13; 3.0 g, 20.0 mmol) in THF (70 mL) at 0 C was added Et3N (10.12 g, 14.0 mL, 100 mmol). The reaction mixture was stirred at 0 C for 10 min. After that, PhCOCl (3.93 g, 3.25 mL, 28.0 mmol) was added and the reaction mixture was stirred at 0 C for 45 min. After the reaction was finished, THF was evaporated. The residue obtained after evaporation of the solvent was subjected to column chromatography (30 g silica gel, n-hexane/EtOAc 4:1). The obtained compound was further crystallized from CH2Cl2/n-hexane to afford aldehyde 15. Yield: 4.88 g (98%), white crystals, Rf 0.56 (n-hexane/Me2CO 2:1), m.p. 68-71 C (CH2Cl2/n-hexane). Anal. Calcd for C16H11NO2 requires: C, 77.10; H, 4.45; N, 5.62. Found: C, 76.77; H, 4.69; N, 5.41. MS (EI), m/z (%): 249 [M]+ (43), 105 [C6H5C=O]+ (100), 77 [C6H5]+ (79). IR (CHCl3) max: 3026, 1686 (C=O), 1673 (C=O), 1440, 706 cm-1. 1H NMR (400 MHz, CDCl3) 10.05 (s, 1H, CHO), 8.32-8.30 (m, 1H, H-7), 8.12-8.10 (m, 1H, H-4), 7.94 (s, 1H, H-2) , 7.78-7.76 (m, 2H, H-2, H-6), 7.70-7.66 (m, 1H, H-4), 7.62-7.56 (m, 2H, H-3, H-5), 7.49-7.42 (m, 2H, H-5, H-6). 13C NMR (100 MHz, CDCl3) 185.8 (CHO), 168.5 (C=O), 137.6 (C-2), 136.8 (C-1), 133.0 (C-4), 129.4 (C-2, C-6), 129.3 (C-7a), 129.0 (C-3, C-5), 126.6 (C-6), 126.2 (C-3a), 125.6 (C-5), 122.2 (C-3), 122.0 (C-4), 116.1 (C-7).
References:
Budovská, Mariana;Pilátová, Martina Bago;Tischlerová, Viera;Moj?i?, Ján [Arkivoc,2016,vol. 2016,# 6,p. 198 - 234]
487-89-8
598 suppliers
$6.00/10g
591-50-4
473 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry

27092-42-8
9 suppliers
inquiry
1496-76-0
6 suppliers
inquiry

27092-42-8
9 suppliers
inquiry