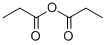
N-(1-Oxopropyl)butanamide synthesis
- Product Name:N-(1-Oxopropyl)butanamide
- CAS Number:32796-69-3
- Molecular formula:C7H13NO2
- Molecular Weight:143.18
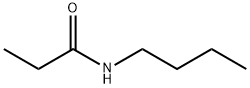
2955-67-1
36 suppliers
$25.00/5g

32796-69-3
1 suppliers
inquiry
Yield:32796-69-3 92%
Reaction Conditions:
with potassium peroxymonosulfate sulfate;water;potassium bromide in dichloromethane at 20; for 7 h;Sealed tube;Irradiation;
Steps:
N-Propionylbenzamide (B1); Typical Procedure
General procedure: N-Propylbenzamide (A1) (40.8 mg, 0.25 mmol, 1.0 equiv), Oxone(307.8 mg, 0.50 mmol, 2.0 equiv), KBr (8.9 mg, 0.075 mmol, 0.3equiv), H2O (198.2 mg, 44 equiv, 0.2 mL) and CH2Cl2 (1.5 mL) wereadded to a 15 mL sealed tube containing a magnetic stir bar. The reaction mixture was stirred at room temperature for 7 hours under irradiation with an 8 W white LED. After completion of the reaction, saturated Na2SO3 (5.0 mL) was added and the mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organics were washed with brine(10 mL), dried over Mg2SO4, filtered and concentrated. The residue was purified by flash column chromatography on silica gel (EtOAc/PE,1:6) to afford N-propionylbenzamide (B1). 3b White solid; yield: 37.7 mg (85%); mp 93-94 °C (Lit. 3b 93-94 °C).1 H NMR (400 MHz, CDCl 3 ): δ = 8.54 (br s, 1 H), 7.84 (d, J = 7.2 Hz, 2 H),7.61 (t, J = 7.6 Hz, 1 H), 7.51 (t, J = 8.0 Hz, 2 H), 3.04 (q, J = 7.2 Hz, 2 H),1.23 (t, J = 7.6 Hz, 3 H).13 C NMR (100 MHz, CDCl 3 ): δ = 177.8, 166.0, 133.3, 133.0, 129.1,128.0, 31.5, 8.4.
References:
Mei, Chong;Hu, Yixin;Lu, Wenjun [Synthesis,2018,vol. 50,# 15,art. no. SS-2018-C0114-ST,p. 2999 - 3005]
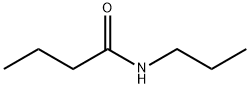
5129-73-7
1 suppliers
inquiry

32796-69-3
1 suppliers
inquiry