N-(1H-INDOL-7-YL)-2-PHENOXYACETAMIDE synthesis
- Product Name:N-(1H-INDOL-7-YL)-2-PHENOXYACETAMIDE
- CAS Number:737801-80-8
- Molecular formula:C16H14N2O2
- Molecular Weight:266.29
5192-04-1
151 suppliers
$30.00/100mg

501-53-1
392 suppliers
$10.00/1g

737801-80-8
1 suppliers
inquiry
Yield:737801-80-8 96.7%
Reaction Conditions:
with sodium hydroxide in dichloromethane;water at 10; for 2 h;Product distribution / selectivity;
Steps:
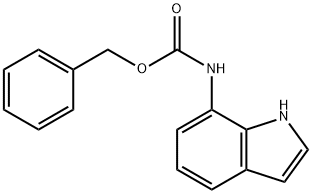
15
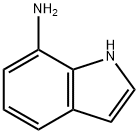
(1H-Indol-7-yl)-carbamic acid benzyl ester Equip a 12-L reaction flask with a cooling bath, air driven stirring apparatus, addition funnel, and thermometer probe. Thoroughly purge the flask with nitrogen, charge 7- aminoindole (352 g, 2.663 moles), CH2C12 (5.30 L, 15 volumes) and 2N NaOH (1. 76 L, 3.515 moles). After cooling the biphasic solution to less than 10 °C, benzyl chloroformate (500 g, 2.929 moles) add dropwise at such a rate so as to maintain the temperature at less than 10 °C over one hour. Stir the reaction vigorously for 1 hour until complete by TLC. Separate the layers and extract the aqueous layer with CH2C12 (1.7 L).
References:
WO2005/92854,2005,A1 Location in patent:Page/Page column 57