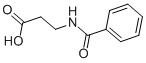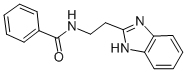
N-[2-(1H-BENZOIMIDAZOL-2-YL)-ETHYL]-BENZAMIDE synthesis
- Product Name:N-[2-(1H-BENZOIMIDAZOL-2-YL)-ETHYL]-BENZAMIDE
- CAS Number:107313-47-3
- Molecular formula:C16H15N3O
- Molecular Weight:0
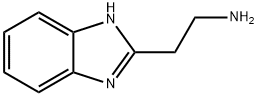
29518-68-1
98 suppliers
$22.00/250mg

98-88-4
574 suppliers
$10.00/5g
![N-[2-(1H-BENZOIMIDAZOL-2-YL)-ETHYL]-BENZAMIDE](/CAS/GIF/107313-47-3.gif)
107313-47-3
8 suppliers
inquiry
Yield:107313-47-3 76%
Reaction Conditions:
with triethylamine in tetrahydrofuran; for 3 h;Reflux;
Steps:
4.1.6. General procedures for the acylation of (1H-benzimidazole-2-yl)alkylamines
General procedure: Starting amino compound (0.238 mmol) was dissolved in THF (25 mL) and triethylamine (10 drops) was added to the stirred solution. Acyl halide (0.149 mmol) was added dropwise to the stirred solution, which was then heated under reflux for 3h. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in EtOAc and washed with aq. K2CO3 solution followed by brine. The organic layer was then dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography eluting with 1-5 methanol/DCM v/v.
References:
Elshihawy, Hosam;Helal, Mohamed A.;Said, Mohamed;Hammad, Mohamed A. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 1,p. 550 - 558]

98-88-4
574 suppliers
$10.00/5g
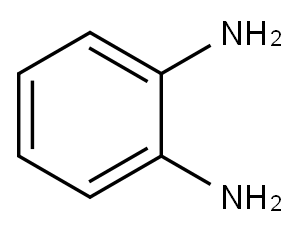
95-54-5
496 suppliers
$9.00/1g
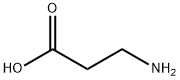
107-95-9
728 suppliers
$5.00/10g
![N-[2-(1H-BENZOIMIDAZOL-2-YL)-ETHYL]-BENZAMIDE](/CAS/GIF/107313-47-3.gif)
107313-47-3
8 suppliers
inquiry

95-54-5
496 suppliers
$9.00/1g
![N-[2-(1H-BENZOIMIDAZOL-2-YL)-ETHYL]-BENZAMIDE](/CAS/GIF/107313-47-3.gif)
107313-47-3
8 suppliers
inquiry