N-(2,5-dimethoxyphenyl)-4-nitrobenzenesulfonamide synthesis
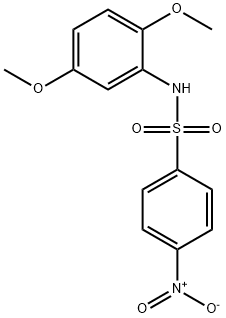
- Product Name:N-(2,5-dimethoxyphenyl)-4-nitrobenzenesulfonamide
- CAS Number:331240-68-7
- Molecular formula:C14H14N2O6S
- Molecular Weight:338.34
Yield:331240-68-7 78%
Reaction Conditions:
with pyridine in dichloromethane at 20;
Steps:
2 Example 2 N-(2,5-dimethoxyphenyl)-4-nitrobenzenesulfonamide (9)
Example 2
N-(2,5-dimethoxyphenyl)-4-nitrobenzenesulfonamide (9)
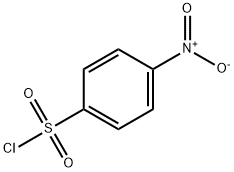
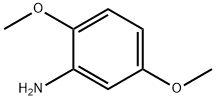
To a solution of 2,5-dimethoxyaniline (4.87 g, 31.59 mmol) in DCM (175 mL) were subsequently added dropwise pyridine (2.56 mL, 31.59 mmol) and a solution of 4-nitrobenzenesulfonyl chloride (7 g, 31.59 mmol) in DCM.
After 24 hours of stirring at room temperature, the reaction mixture was quenched with H2O.
After extraction three times with DCM, the organic layer was washed with an aqueous solution of 10% K2CO3, and a saturated aqueous solution of NaCl.
After drying with MgSO4, filtration and concentration under vacuum, the crude was purified by chromatography over silica gel (PE/AcOEt: 7/3) and afforded the expected compound (9) as a yellow solid (7,8 g, 23 mmol) with 78 % yield. (Rf = 0.88 (EP/EtOAc: 1/1)); mp = 165 °C. RMN 1H (300 MHz, CDCl3): 8.24 (d, 2H, H1-H4), 7.94 (d, 2H, H2-H3), 7.17 (d, 1H, H6), 7.06 (s, 1H, H5), 6.67 (dd, 1H, H8), 6.61 (d, 1H, H7), 3.77 (s, 3H, CH3), 3.59 (s, 3H, CH3). RMN 13C (75 MHz, CDCl3): 159.0 (CIV), 150.2 (CIV), 144.8 (CIV), 143.9 (CIV), 128.5 (C2-C3), 125.4 (CIV), 124.0 (C1-C4), 111.5 (C8), 110.8 (C7), 108.3 (C6), 56.7 (CH3), 55.8 (CH3). HRMS:
Calculated for [M+Na]+ 361,0470; Measured: 361.0470. IR: 3310 (v N-H), 3107 (v Car-H), 2841 (v OC-H), 1534 (vas NO2), 1391 (vs NO2), 1345 (vas SO2), 1157 (vas NO2)
References:
EP3412652,2018,A1 Location in patent:Paragraph 0065-0066
89-39-4
101 suppliers
$46.00/5g

331240-68-7
3 suppliers
inquiry

150-78-7
512 suppliers
$5.00/25g

331240-68-7
3 suppliers
inquiry