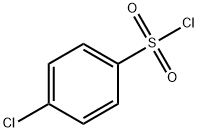
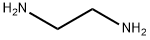N-(2-aminoethyl)-4-chlorobenzenesulfonamide synthesis
- Product Name:N-(2-aminoethyl)-4-chlorobenzenesulfonamide
- CAS Number:83019-90-3
- Molecular formula:C8H11ClN2O2S
- Molecular Weight:234.7
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 18 h;
Steps:
4.1.1. General procedure for synthesis of N-(2-aminoethyl and 3-aminopropyl)unsubstituted and substituted phenyl sulfonamide (3a-p)
General procedure: A solution of appropriate sulfonyl chloride (2a-h, 5 mmol) in dichloromethane (2 mL) was added dropwise to a solution of properdiamine (1a,b, 10 mmol) and trimethylamine (300 mg, 30 mmol) in dichloromethane (20 mL) at 0 °C. The reaction mixture was allowed to stir at rt for 18 h. The reaction mixture was washed with saturated solution of NaHCO3 (20 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated under vacuum to give the titled products 3a-p [27].
References:
Abdel-Maksoud, Mohammed S.;Ammar, Usama M.;El-Gamal, Mohammed I.;Gamal El-Din, Mahmoud M.;Mersal, Karim I.;Ali, Eslam M.H.;Yoo, Kyung Ho;Lee, Kyung-Tae;Oh, Chang-Hyun [Bioorganic Chemistry,2019,vol. 93,art. no. 103349]