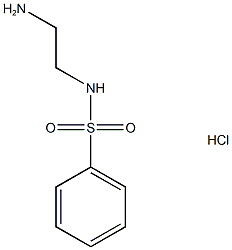
N-(2-AMINOETHYL)BENZENESULFONAMIDE HYDROCHLORIDE synthesis
- Product Name:N-(2-AMINOETHYL)BENZENESULFONAMIDE HYDROCHLORIDE
- CAS Number:53672-99-4
- Molecular formula:C8H13ClN2O2S
- Molecular Weight:236.719

222639-98-7
20 suppliers
$92.50/25mg

53672-99-4
20 suppliers
inquiry
Yield:53672-99-4 99%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;dichloromethane at 20; for 16 h;
Steps:
The Boc-protected compound (0.17g, 0.58 mmol) was then dissolved in dry DCM (7mL).1 mL (excess) of HCl in 4.0M dioxane was added dropwise. The mixture was then stirred at room temperature for 16h when TLC analysis (DCM:MeOH; 9:1) showed complete consumption of starting material. After concentrated in vacuo, the solid obtained was washed with diethyl ether and dried under vacuum overnight to afford the title sulfonamide as a white solid (0.32g, 99 %). The M. p.: 179.2-179.9, max (ATR): (N- H): 3000, 1677, 1632, 1540 cm-1. δH (400 MHz, D2O): 7.75 (m, 2H, Ar-H), 7.61 (m, 1H, Ar-H), 7.53 (m, 2H, Ar-H), 3.05 (d, 2H, J=6.2 Hz, CH2), 2.99 (d, 2H, J=6.2 Hz, CH2). δC (D2O, 400 MHz): 137.4 (C-Ar), 133.8 (C-Ar), 129.9 (C-Ar), 126.6 (C-Ar), 39.9 (CH2), 39.1 (CH2). LRMS (ES+): m/z 201 (M+H), HRMS (ASAP+): Found M+H 201.0698, C8H13N2O2S, requires M 201.0697.
References:
WO2022/136838,2022,A1 Location in patent:Page/Page column 40
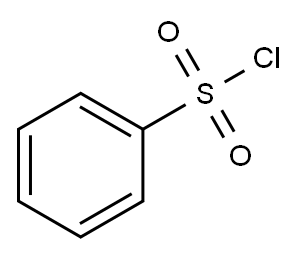
98-09-9
337 suppliers
$12.00/25g

53672-99-4
20 suppliers
inquiry