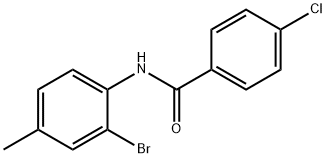
N-(2-bromo-4-methylphenyl)-4-chlorobenzamide synthesis
- Product Name:N-(2-bromo-4-methylphenyl)-4-chlorobenzamide
- CAS Number:299954-67-9
- Molecular formula:C14H11BrClNO
- Molecular Weight:324.6

299954-67-9
5 suppliers
$130.71/250MG
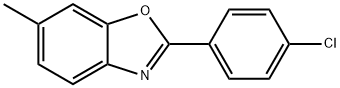
16155-97-8
1 suppliers
inquiry
Yield:16155-97-8 90%
Reaction Conditions:
with copper(II) ferrite;potassium carbonate in dimethyl sulfoxide at 120; for 24 h;Schlenk technique;Inert atmosphere;Sealed tube;
Steps:
General procedure for synthesis of substituted benzoxazoles
General procedure: A 25 mL Schlenk tube equipped with a magnetic stirring bar was charged withCuFe2O4 nanoparticles (0.05 mmol, 12 mg), substituted substitutedN-(2-bromophenyl)benzamides (1) (0.5 mmol), K2CO3 (1.0 mmol, 139 mg). Thetube was evacuated twice and backfilled with nitrogen, and DMSO (1.5 mL) wasadded to the tube under nitrogen atmosphere. The tube was sealed and then themixture was allowed to stir under nitrogen atmosphere at 90°C-120°C for 24 h. After completion of the reaction, the resulting solution was cooled to room temperature, and the solvent was removed with the aid of a rotary evaporator. The residue was purified by column chromatography on silica gel using petroleum ether/ethyl acetate as eluentto provide the desired product
References:
Yang, Daoshan;Zhu, Xiao;Wei, Wei;Jiang, Min;Zhang, Ning;Ren, Dandan;You, Jinmao;Wang, Hua [Synlett,2014,vol. 25,# 5,art. no. ST-2013-W1096-L,p. 729 - 735] Location in patent:supporting information