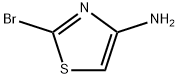
N-(2-bromo-4-thiazolyl)acetamide synthesis
- Product Name:N-(2-bromo-4-thiazolyl)acetamide
- CAS Number:7041-82-9
- Molecular formula:C5H5BrN2OS
- Molecular Weight:221.07

41731-35-5
67 suppliers
$80.00/100mg

75-36-5
561 suppliers
$17.92/100G

7041-82-9
2 suppliers
inquiry
Yield:7041-82-9 29.4%
Reaction Conditions:
Stage #1: 2‐bromo‐1,3‐thiazol‐4‐amine hydrobromidewith triethylamine in dichloromethane at 0 - 20; for 0.25 h;Inert atmosphere;
Stage #2: acetyl chloride in dichloromethane at 20;
Steps:
4.27.1 Step 1 N-(2-bromothiazol-4-yl)acetamide (ZSL-000466-082).
To a suspension of 2-bromothiazol-4-amine hydrogen bromide (1 g, 3.87 mmol) in DCM (5 mL) was added dropwise TEA (2.1 mL, 15.48 mmol) at 0 oC. After the addition, the reaction mixture was stirred at r.t for 15 min. then acetyl chloride (450 mg, 5.81 mmol) was added. The reaction mixture was stirred at r.t overnight. The reaction mixture was quenched with water (10 mL). The mixture was neutralized with saturated aq. NaHCO3 solution and extracted with EA. Combined organic layers were washed with water and brine, dried over Na2SO4 and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by chromatography (eluted with PE: EA = 10:1 to 3:1) to afford N-(2-bromothiazol-4-yl)acetamide (250 mg, 29.4% yield) as a white solid. Retention time (LC-MS): 1.930 min. MH+ 221.
References:
WO2016/44792,2016,A1 Location in patent:Page/Page column 120; 121