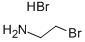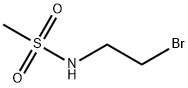
N-(2-bromoethyl)methanesulfonamide synthesis
- Product Name:N-(2-bromoethyl)methanesulfonamide
- CAS Number:63132-74-1
- Molecular formula:C3H8BrNO2S
- Molecular Weight:202.07
Yield:63132-74-1 69%
Reaction Conditions:
with triethylamine in dichloromethane;
Steps:
F.25 N-(2-Bromoethyl)methanesulphonamide
Description 14 (intermediate for Example 25) N-(2-Bromoethyl)methanesulphonamide A stirred solution of 2-bromoethylamine hydrobromide (5.10 g, 0.025 mole) and triethylamine (6.96 g, 0.050 mole) in dichloromethane (200 ml) at ice bath temperature was treated dropwise with methanesulphonyl chloride (1.96 ml, 0.025 mole). The mixture was allowed to warm to room temperature and stir for 16 h, then washed with water and 5M HCl acid, dried (Na2 SO4) and concentrated in vacuo to afford the title compound (D14) as a colourless oil which solidified on standing to give a white solid (3.5 g, 69%). 1 H NMR (CDCl3); δ: 4.92(s,1H), 3.62-3.48(m,4H), 3.05(s,3H).
References:
US5998409,1999,A
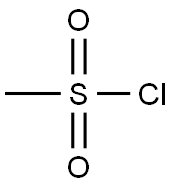
124-63-0
6 suppliers
$10.00/5g

63132-74-1
12 suppliers
inquiry