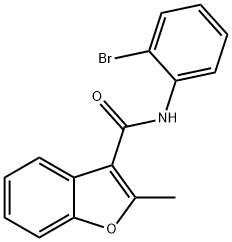
N-(2-BroMophenyl)-2-Methylbenzofuran-3-carboxaMide synthesis
- Product Name:N-(2-BroMophenyl)-2-Methylbenzofuran-3-carboxaMide
- CAS Number:1373243-63-0
- Molecular formula:C16H12BrNO2
- Molecular Weight:330.18
Yield:1373243-63-0 64%
Reaction Conditions:
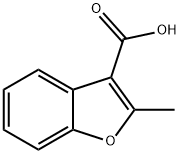
Stage #1: 2-methyl-1-benzofuran-3-carboxylic acidwith thionyl chloride;triethylamine in dichloromethane at 0 - 20; for 6 h;
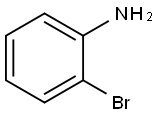
Stage #2: 2-bromoaniline in dichloromethane at 20; for 16 h;
Steps:
161
Intermediate- 161 : N-(2-Bromophenyl)-2-methylbenzofuran-3 -carboxamide :To solution of Intermediate- 160 (1 g, 5.68 mmol) in DCM (30.0 mL) was added tnethylamine (2.9 g, 4.0 mL, 28.4 mmol) and thionylchloride (2.50 mL) at 0 °C. The reaction mixture was stirred at ambient temperature for 6 h and poured into CH2CI2 (30.0 mL). The mixture was washed with 10 % HCI, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo to dryness. The crude product was dissolved DCM (30.0 mL) followed by the addition of 2-bromoaniline (4.42 g, 25.7 mmol). The reaction mixture was stirred at ambient temperature for 16 h and the solvent was removed under vacuo. To the residue was added ethyl acetate (50 mL) and water 20 (mL). The layers were separated and the organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo to dryness. The residue was subjected to column chromatography eluting with ethyl acetate/hexane (20 %) to give the title compound (1.2 g, 64 %) as a white solid.
References:
WO2012/49555,2012,A1 Location in patent:Page/Page column 132