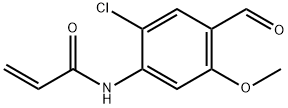
N-(2-chloro-4-formyl-5-methoxyphenyl)acrylamide synthesis
- Product Name:N-(2-chloro-4-formyl-5-methoxyphenyl)acrylamide
- CAS Number:1251456-87-7
- Molecular formula:C11H10ClNO3
- Molecular Weight:239.65

145742-50-3
34 suppliers
$34.00/100mg
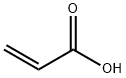
79-10-7
733 suppliers
$20.50/500ml

1251456-87-7
23 suppliers
$371.00/1g
Yield:1251456-87-7 71%
Reaction Conditions:
with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;triethylamine in ethyl acetate at 25 - 40; for 1.5 h;
Steps:
C.1
Step C: Preparation of N-[2-chloro-4-formyl-5-(methyloxy)phenyl]-2-propenamidePreparation 1Acrylic acid (commercially available, for example, from Aldrich) (46 ml, 0.67 mol) was added slowly to a stirred suspension of 4-amino-5-chloro-2-methoxybenzaldehyde (Step B) (50.0 g, 0.27 mol) and triethylamine (204 g, 2.02 mol) in ethyl acetate (0.85 L) at 25° C. Propanephosphonic anhydride (50% in ethyl acetate; 429 g, 0.67 mol) was added over 30 minutes keeping reaction temperature at 30-40° C. The mixture was stirred at 30-40° C. for a further 1 hour and then cooled to 25° C. and diluted with water (0.26 L) and acidified with 32% hydrochloric acid (108 g) to pH 2-3. The organic layer was separated and washed with a mixture of water (0.23 L) and 32% sodium hydroxide (14 g)-aqueous layer ca. pH 7. The organic phase was washed with water (0.23 L) and then concentrated under reduced pressure (ca. 300 mbar) to remove 0.56 kg of distillate. Methylcyclohexane (335 g) was added and then a further 286 g of distillate was removed under reduced pressure. Methylcyclohexane (111 g) was added and then the resulting suspension was cooled to 20° C., filtered and washed with methylcyclohexane. The cake was dried at 40° C. under reduced pressure for 12 hours to give N-[2-chloro-4-formyl-5-(methyloxy)phenyl]-2-propenamide (46 g, 71%)
References:
US2012/46469,2012,A1 Location in patent:Page/Page column 4
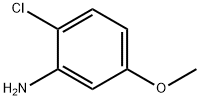
2401-24-3
230 suppliers
$8.00/1g

1251456-87-7
23 suppliers
$371.00/1g

145742-50-3
34 suppliers
$34.00/100mg

1251456-87-7
23 suppliers
$371.00/1g