N'-(2-CHLOROACETYL)-4-METHYLBENZOHYDRAZIDE synthesis
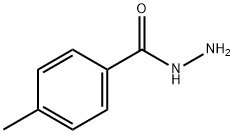
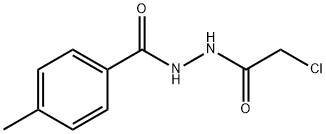
- Product Name:N'-(2-CHLOROACETYL)-4-METHYLBENZOHYDRAZIDE
- CAS Number:199938-21-1
- Molecular formula:C10H11ClN2O2
- Molecular Weight:226.66
Yield:199938-21-1 82%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20; for 16 h;Product distribution / selectivity;
Steps:
35.b; 44.b
To a mixture of 4-methylbenzohydrazide 14 (1O g, 66.59 mmol) and K2CO3 (16.57 g, 119.86 mmol) in acetonitrile (100 mL) was added 2-chloroacetyl chloride (9.02 g, 79.9 mmol) dropwise at ambient temperature. The resulting mixture was stirred at ambient temperature for 16 hours. The solid was collected, washed with H2O and ether, and dried to afford N'-(2-chloroacetyl)-4- methylbenzohydrazide 15 (13 g, 82%) which was used for the next step without further purification. A mixture of N'-(2-chloroacetyl)-4-methylbenzohydrazide 15 (6.0 g, 26.47 mmol) and phosphorous oxychloride (5.0 g, 32.84 mmol) in acetonitrile (20 mL) was stirred under reflux for 16 hours and concentrated. The residue was subjected to flush column chromatography eluting with 10-25% acetone/hexanes to give compound 16 (4 g). Yield: 72%. 1H-NMR (300 MHz, CHLOROFORM-d) δ (ppm): 7.96 (d, 2 H), 7.30 (d, 2 H), 4.77 (s, 2 H), 2.44 (s, 3 H).
References:
WO2010/139966,2010,A1 Location in patent:Page/Page column 91-92; 102-103

99-94-5
592 suppliers
$9.00/1g

199938-21-1
10 suppliers
inquiry

94-08-6
224 suppliers
$6.00/5g

199938-21-1
10 suppliers
inquiry