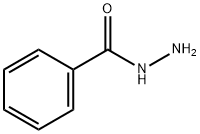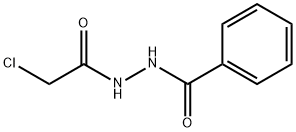
N'-(2-CHLOROACETYL)BENZOHYDRAZIDE synthesis
- Product Name:N'-(2-CHLOROACETYL)BENZOHYDRAZIDE
- CAS Number:50677-24-2
- Molecular formula:C9H9ClN2O2
- Molecular Weight:212.63
Yield:50677-24-2 88.11%
Reaction Conditions:
with sodium hydroxide in acetonitrile at 0; for 1 h;
Steps:
N'-(2-chloroacetyl)benzohydrazide(9).
Benzohydrazide (5.00g, 36.76mmol) was added to acetonitrile (30mL) in a two-necked flask, and acetonitrile (15mL) solution of 2-chloroacetyl chloride (4.77g, 42.23mmol) was added dropwise to the two-necked flask using a constant pressure dropping funnel under ice bath, at the same time, 50% NaOH (1.75g, 43.70mmol) solution was added dropwise to the reaction solution. The resulted mixture was stirred at 0 for 1h and detected by TLC which was concentrated under reduced pressure. Water was added and the mixture was extracted with CH2Cl2. Organic layers were combined, dried over MgSO4, filtered, and concentrated to get 9 as white foamy solid (6.88g, yield: 88.11 %). 1H NMR (300MHz, DMSO-d6) δ 10.50 (s, 1H), 10.41 (s, 1H), 7.79 - 7.91 (m, 2H), 7.57 - 7.64 (m, 1H), 7.40 - 7.56 (m, 2H), 4.19 (s, 2H).
References:
Fang, Lincheng;Tian, Jiping;Zhang, Kaixuan;Zhang, Xiaoyi;Liu, Yingqiao;Cheng, Zhibo;Zhou, Jinpei;Zhang, Huibin [Bioorganic and Medicinal Chemistry,2021,vol. 46,art. no. 116370]
65-85-0
1336 suppliers
$10.00/25g

50677-24-2
19 suppliers
inquiry
93-89-0
505 suppliers
$13.00/100g

50677-24-2
19 suppliers
inquiry
92789-04-3
0 suppliers
inquiry

50677-24-2
19 suppliers
inquiry