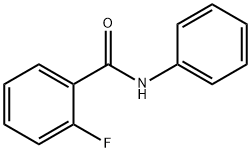
N-(2-Chlorophenyl)-2-fluorobenzaMide, 97% synthesis
- Product Name:N-(2-Chlorophenyl)-2-fluorobenzaMide, 97%
- CAS Number:1629-11-4
- Molecular formula:C13H9ClFNO
- Molecular Weight:249.67
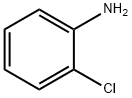
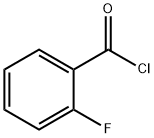
1747-80-4
13 suppliers
$110.00/50mg

1629-11-4
6 suppliers
inquiry
Yield:1629-11-4 55%
Reaction Conditions:
with iodosylbenzene;anhydrous zinc chloride in 2,2,2-trifluoroethanol at 45; for 3 h;Schlenk technique;Green chemistry;
Steps:
3.2. general procedure for the synthesis of 2 and 3
General procedure: A Schlenk tube with a magnetic stir bar was charged withamide 1 (0.50 mmol), ZnCl2 (0.50 mmol, 68.0 mg), 2,2,2-trifluoroethanol (2.0 mL). Then, PhI=O (0.60 mmol, 132.0mg) was added. The reaction mixture was then heated to 45oC and stirred for indicated hours. The reaction mixture wasthen allowed to cool to ambient temperature, and all of thevolatiles were removed under vacuum; the crude productwas purified on flash chromatography, eluting with petroleum/ethyl acetate, to provide products 2 or 3.
References:
Liu, Haixuan;Sha, Qiang [Letters in Organic Chemistry,2022,vol. 19,# 3,p. 173 - 180]