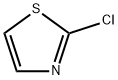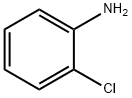
N-(2-Chlorophenyl)thiazol-2-amine synthesis
- Product Name:N-(2-Chlorophenyl)thiazol-2-amine
- CAS Number:136343-79-8
- Molecular formula:C9H7ClN2S
- Molecular Weight:210.68
Yield:136343-79-8 95%
Reaction Conditions:
with toluene-4-sulfonic acid in isopropyl alcohol at 80; for 48 h;
Steps:
General procedure for nucleophilic substitution reactions towards compounds 3 - 12
General procedure: 1.0 equiv of the halothiazole, 1.5 equiv of the amino-substrate and 0.5 equiv of p-toluenesulfonic acid were dissolved in i-propanol and the reaction mixture was stirred at 80 °C until the reaction was complete (TLC). The reaction mixture was then diluted with ethyl acetate and washed with saturated NaHCO3 solution and brine followed by general work-up. Purification was carried out by Kugelrohr distillation.
References:
Schnürch, Michael;Waldner, Birgit;Hilber, Karlheinz;Mihovilovic, Marko D. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 7,p. 2149 - 2154] Location in patent:experimental part