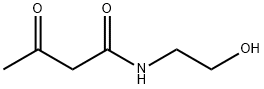
N-(2-hydroxyethyl)acetoacetamide synthesis
- Product Name:N-(2-hydroxyethyl)acetoacetamide
- CAS Number:24309-97-5
- Molecular formula:C6H11NO3
- Molecular Weight:145.16
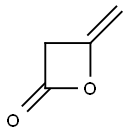
674-82-8
206 suppliers
inquiry

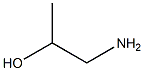
141-43-5
867 suppliers
$9.00/10g

24309-97-5
47 suppliers
$14.00/100mg
Yield:24309-97-5 80%
Reaction Conditions:
in tetrahydrofuran at -5 - 0; for 1 h;
Steps:
AL AL. N-(2-Hydroxyethyl)-3-oxobutanamide
AL. N-(2-Hydroxyethyl)-3-oxobutanamide
23 ml (0.38 mmol) of ethanolamine were added dropwise to a solution of 30 g of diketene (0.36 mol) in 300 ml tetrahydrofuran at -5 to 0° C.
After 1 h stirring at 0° C. no more starting material was detected by thin layer chromatography.
The reaction mixture was evaporated and the residue purified by column chromatography.
This gave 41.44 g (0.29 mol, 80% yield) as a white solid.
IR (neat, cm-1): 3273, 3097, 2973, 2938, 2880, 1710, 1646, 1558, 1494, 1465, 1420, 1362, 1347, 1312, 1297, 1215, 1190, 1166, 1052, 1038.
CHN-Elemental analysis: C, 49.13; H, 7.76; N, 9.71.
LC-MS: 145 (M), 127 (M-H2O).
1H-NMR (CDCl3, 400 MHz): δ [ppm]=7.5 (1H), 3.7 (2H), 3.4 (4H), 2.2 (3H).
References:
US2013/109662,2013,A1 Location in patent:Paragraph 0394; 0395; 0396; 0397; 0398; 0399
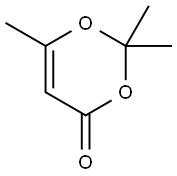
5394-63-8
225 suppliers
$19.00/25g

141-43-5
867 suppliers
$9.00/10g

24309-97-5
47 suppliers
$14.00/100mg