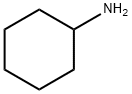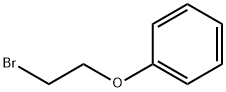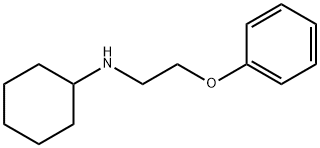
N-(2-phenoxyethyl)cyclohexanamine synthesis
- Product Name:N-(2-phenoxyethyl)cyclohexanamine
- CAS Number:356532-64-4
- Molecular formula:C14H21NO
- Molecular Weight:219.32
Yield:356532-64-4 68%
Reaction Conditions:
with potassium carbonate;sodium iodide in ethanol; for 12 h;Reflux;
Steps:
11
2-(Bromoethoxy)benzene (43.0 g (0.215 mol)) and cyclohexylamine (53.0 g (0.54 mol)) were dissolved in ethanol (400 mL), and then potassium carbonate (100.0 g (0.72 mol)) and sodium iodide (30.0 g (0.16 mol)) were added thereto. The mixture was stirred for 12 hours under heating at reflux. After the reaction mixture was returned to room temperature, the insoluble fraction was removed by filtration and the solvent was distilled away under reduced pressure. The residue was purified by a silica gel chromatography (petroleum ether : ethyl acetate = 10 : 1) to obtain N-(2-phenoxyethyl) cyclohexylamine (compound-02) (32.0 g, 0.15 mol, yield 68%).
References:
EP2357165,2011,A1 Location in patent:Page/Page column 19
626-62-0
138 suppliers
$10.00/1g
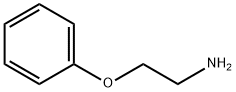
1758-46-9
193 suppliers
$6.00/250mg

356532-64-4
5 suppliers
inquiry

108-95-2
744 suppliers
$14.00/25g

356532-64-4
5 suppliers
inquiry