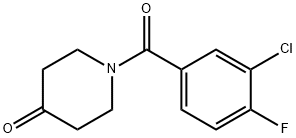
N-(3-chloro-4-fluorobenzoyl)-piperidine-4-one synthesis
- Product Name:N-(3-chloro-4-fluorobenzoyl)-piperidine-4-one
- CAS Number:223632-64-2
- Molecular formula:C12H11ClFNO2
- Molecular Weight:255.67
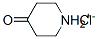
41979-39-9
115 suppliers
$15.00/10mg

65055-17-6
132 suppliers
$9.00/1g

223632-64-2
26 suppliers
inquiry
Yield:223632-64-2 85%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1.25 h;Inert atmosphere;
Steps:
1.2 Step 2: 1-[(3-chloro-4-fluorophenyl)carbonyl]piperidin-4-one
To a solution of piperidin-4-one hydrochloride (11.6 g, 86.3 mmol, 1.10 equiv) in dichloromethane (120 mL) under an inert atmosphere of nitrogen was added triethylamine (12.1 g, 120 mmol, 2.00 equiv)) at 0° C. This was followed by the addition of a solution of 3-chloro-4-fluorobenzoyl chloride [Example 1, Step 1] (15 g, 78.5 mmol, 1.00 equiv) in DCM (50 mL) dropwise with stirring at 0° C. in 15 min. The reaction solution was stirred for 1 h at room temperature. The reaction was quenched with water (200 mL). The resulting solution was extracted with dichloromethane (3×200 mL), and the organic layers were combined. The organic layers were washed with water (3×100 mL) and brine (3×100 mL). The mixture was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:2) to afford 17 g (85%) of 1-[(3-chloro-4-fluorophenyl)carbonyl]piperidin-4-one as a white solid. LC-MS: m/z=256[M+H]+.
References:
US2018/79742,2018,A1 Location in patent:Paragraph 0308; 0309

65055-17-6
132 suppliers
$9.00/1g

223632-64-2
26 suppliers
inquiry
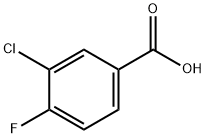
403-16-7
274 suppliers
$6.00/5g

223632-64-2
26 suppliers
inquiry
![1,4-Dioxa-8-azaspiro[4.5]decane](/CAS/GIF/177-11-7.gif)
177-11-7
283 suppliers
$6.00/1g

223632-64-2
26 suppliers
inquiry