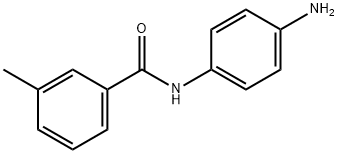
N-(4-AMINOPHENYL)-3-METHYLBENZAMIDE synthesis
- Product Name:N-(4-AMINOPHENYL)-3-METHYLBENZAMIDE
- CAS Number:425651-25-8
- Molecular formula:C14H14N2O
- Molecular Weight:226.27
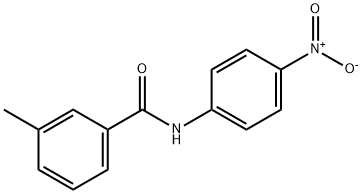
73230-98-5
4 suppliers
$30.00/20mg

425651-25-8
18 suppliers
$45.00/10mg
Yield:425651-25-8 85.2%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water; for 2 h;Reflux;
Steps:
16.2 2. Preparation of 3-methyl-N-(4-aminophenyl)benzamide (11j)
Compound 10j was dissolved in EtOH (20 ml), and an aqueous solution of iron powder (2.5 g) and ammonium chloride (0.53 g, 10 mmol) was added and the reaction was refluxed for 2 hours. Followed by hot filter, collected filtrate spin dry. The resulting solid was dissolved in acetone and the filtrate was collected by filtration and the solution was dried to give compound 11j in 85.2% yield.
References:
CN106699755,2017,A Location in patent:Paragraph 0105; 0285; 0286
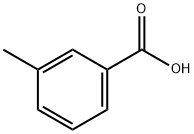
99-04-7
463 suppliers
$8.00/100g

425651-25-8
18 suppliers
$45.00/10mg
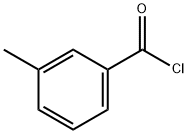
1711-06-4
324 suppliers
$6.00/25g

425651-25-8
18 suppliers
$45.00/10mg

100-01-6
36 suppliers
$11.19/25G

425651-25-8
18 suppliers
$45.00/10mg
106-50-3
622 suppliers
$17.00/25g

425651-25-8
18 suppliers
$45.00/10mg