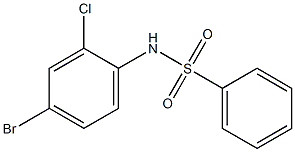
N-(4-Bromo-2-chlorophenyl)benzenesulfonamide, 97% synthesis
- Product Name:N-(4-Bromo-2-chlorophenyl)benzenesulfonamide, 97%
- CAS Number:262433-10-3
- Molecular formula:C12H9BrClNO2S
- Molecular Weight:346.6273
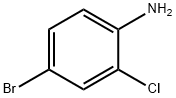
38762-41-3
289 suppliers
$22.00/25g
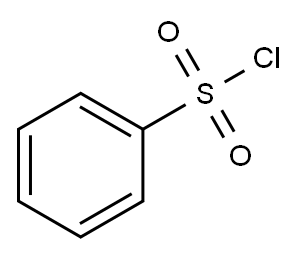
98-09-9
347 suppliers
$12.00/25g

262433-10-3
2 suppliers
inquiry
Yield:262433-10-3 3.2 g (92%)
Reaction Conditions:
with pyridine in dichloromethane;
Steps:
9.a N1-[4-(4-amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2-chlorophenyl]-N1-methyl-1-benzenesulfonamide
a) N1-(4-bromo-2-chlorophenyl)-1-benzenesulfonamide. Benzene sulfonylchloride (2.11 g, 12.0 mmol) was added dropwise under an atmosphere of nitrogen to a stirring solution of 4-bromo-2-chloroaniline (2.06 g, 10.0 mmol), pyridine (3.95 g, 50 mmol) and methylene chloride (15 ml). The mixture was stirred for 3 hours then diluted with ethyl acetate (75 ml) and washed with water (3*20 ml), brine (20 ml) and then dried over MgSO4, filtered and evaporated to give 3.2 g (92%) of N1-(4-bromo-2-chlorophenyl)-1-benzenesulfonamide as an orange solid. 1H NMR (DMSO-d6, 400 MHz) δ 10.10 (1H, s), 7.7 (2H, d), 7.53-7.65 (4H, m), 7.46 (1H, d), 7.18 (1H, d); 13C NMR (DMSO-d6, 100 MHz) δ 140.0, 131.1, 133.0, 132.0, 130.8, 130.3, 129.2, 128.8, 126.6, 119.0; low-resolution MS m/e 346 (M-H+)
References:
US2003/187001,2003,A1